Abstract
The focus of this study is the physicochemical and bacteriological characteristics of groundwater in the Triffa plain, Morocco. In total, 34 groundwater samples were analyzed for major elements (Tp, pH, EC, K+, Na+, Ca2+, Mg2+, Cl−, SO4 2−, NO3 −, NO2 −, NH4 +, H2PO4 −, CO3, and HCO3 −) and trace metal (Al, Cd, Cu, Fe, and Zn) content. The results show that the pH values range between 6.7 and 8.9, electrical conductivity ranges between 740 and 7340 µS/cm, and nitrate content ranges between 1.7 and 212 mg/l. Hydrochemical facies represented using a Piper diagram indicate an Na–K–Cl type water. All the trace metal concentrations are within the admissible standard range except for Cd. The bacteriological analysis showed that the majority of groundwater samples are contaminated. Generally, the content of total coliforms, fecal coliforms, and fecal streptococci ranged from 0 to 140, 0 to 125, and 0 to 108 CFU/100 ml, respectively. The samples are grouped according to three factors. Factor 1 shows strong positive loadings of EC, Mg, Cl, Na and K with 51.91% of total variance (TV); factor 2 shows strong negative loadings of NO3, SO4 and Ca with 17.98% of TV; and factor 3 shows strong negative loading of HCO3 with 15.56 of TV. We conclude that the quality of this groundwater is suitable for irrigation and domestic use (cleaning house, ect).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The deterioration of groundwater quality by nitrate and microbial contamination has been the subject of a number research papers (Lamrani Alaoui et al. 2008; Bahri and Saibi 2012; Douagui et al. 2012; Saber et al. 2013; Merghem et al. 2016; Al-Barakah F et al. 2017; Mallick 2017; Unnisa S and Zainab Bi 2017). The World Health Organization (WHO 2000) has set a limit of 50 mg/l on nitrates in drinking water based on the formation of methemoglobinemia in red cells (Douagui et al. 2012). In Morocco, the local economy is based on agriculture, which consumes 92% of the total water use. Currently, water resources are under serious pressure and agricultural practices often have negative impacts on groundwater quality (Chettouani and Damou 1993; Benkaddour 1997; Fetouani et al. 2008; Fekkoul et al. 2013). The scenarios of possible climate change impact on groundwater in the north of Africa are alarming (IPCC 2007; Jilali 2014). The decrease in the recharge of the aquifers is due to the decrease in precipitation and an increase in temperature (IPCC 2007). Consequently, the groundwater quality is strongly affected (Baba 2012; Jilali et al. 2015a).
Most of the previous studies in this region focused on groundwater quality (nitrate and salinity). This paper illustrates the impacts of irrigated agriculture and wastewater as well as physico-chemical and bacteriological factors and metal content on the quality of groundwater in the unconfined Triffa aquifer in different periods of time. The wastewater of the city of Berkane is rejected in the upstream of the Triffa basin (Cheraa wadi). Piezometric maps show that the direction of groundwater is from SE to NW (Fekkoul et al. 2013). For this reason, most of the contamination takes place in this direction. To assess this, 34 wells have been selected from the entire Triffa plain area (unconfined aquifer) to identify the spatial extent of contamination and evaluate the groundwater quality.
Study area
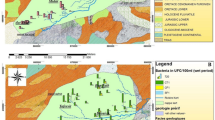
The Triffa aquifer basin is located in the north-eastern part of Morocco and has a surface area of 750 km2. It is limited in the north by the Ouled Mansour hills, in the west by the Kiss and Moulouya rivers, in the south by the Beni Snassen Mountains, and in the east by the Kiss River. It lies between latitudes 35°05′ N and 34°55′ N, and longitudes 2°23′ W and 2°11′ W (Fig. 1). Geologically, the plain is formed by Tertiary and Quaternary formations consisting of alluvial material, silt, sandstone, limestone, and clay (Fetouani et al. 2008). In this region two aquifers are present: (1) an unconfined aquifer hosted by the Tertiary and Quaternary formations; (2) a confined aquifer hosted by the Liasic formation (limestones and dolostones) (DGH 1997; El Mandour 1998; El Idrysy and Smedt 2006, 2007).
Geographical and geological location of the study area (Fetouani et al. 2008), modified. Points numbered in red highlight the location of groundwater samples
The climate of the region is semi-arid and total rainfall does not exceed 327 mm/year. The yearly average temperature is 17.4 °C, but seasonal variability is high, with a minimum of 11 °C and a maximum of 25 °C. The average annual evapotranspiration is about 300 mm/year (Fekkoul et al. 2013).
Materials and methods
Chemical analyses were run on 34 groundwater wells located in different parts of the study area during the months of March, June, and November 2013. Measurements of pH, temperature, electric conductivity (EC) (using ORION STAR A111 and WATER PROOF CC-411), and piezometric level were taken in situ. All water samples were collected in polyethylene bottles and stored in the absence of light at 4 °C. Major ions were analyzed in the laboratory for K+, Na+, Ca2+, Mg2+, Cl−, SO4 2−, NO3 −, NO2 −, NH4 +, H2PO4 − (SKALAR method), and HCO3 − (Rodier 1984). Nitric acid was added to 50 ml of water to produce a 10% nitric acid concentration for the analysis of Al, Cd, Cu, Fe, and Zn, which were determined by atomic emission spectroscopy (ICP-AES; Ultima 2, JobinYvon: equipped with an optical system with two thermo-regulated networks of 4343 and 2400 rev/mm back to back, covering the spectral range 120–800 nm with a nitrogen sweep) in the National Center for Scientific and Technical Research, Rabat-Morocco. A database was then created on the geographic information system (GIS) integrating.
Bacteriological analysis was conducted in the same groundwater wells of the study area in June and November 2013. All measurements were conducted on the same day in the quality control laboratory (University of Mohammed 1st, Morocco). Pathogens analyzed in this study include total coliforms (TC), fecal coliforms (FC), and fecal streptococci (FS: Intestinal Enterococci). Water samples of 100 ml each were filtered (pore size: 0.45 µm) and then transferred onto triphenyl-tetrazolium chloride (TTC) and tergicol lactose agar (AFNOR 1998), which were used as selective media for FC. The FC and TC were counted after 24 h incubation at 44 ± 1 °C. For FS, the selective medium of Slanetz and Bartley was used. The FS were counted after incubation at 37 °C for 24 h. The total number of bacteria was determined as colony forming units per 100 ml (CFU/100 ml).
The principal component analysis (PCA) multivariate statistical technique was used in our research. This method is a quantitative and independent approach for the classification of groundwater samples according to their geochemical characteristics and may simplify and organize large data sets to make useful groupings of similar samples (Kumar et al. 2013; Jilali et al. 2015b). The STATISTICA software was used to process the November 2013 vintage data analyses. For this, the analyses of EC, K+, Na+, Ca2+, Mg2+, HCO3 −, Cl−, SO4 2− and NO3 − were used as variables.
Results and discussion
Groundwater bacteriological quality
Figures 2, 3, and 4 show the abundance of TC, CF, and FS in groundwater sampled in June and November 2013.
-
TC abundance varied from 0 to 140 CFU/100 ml in June and from 0 to 124 CFU/100 ml in November (Fig. 2);
-
FC abundance varied from 0 to 125 CFU/100 ml in June and from 0 to 112 CFU/100 ml in November (Fig. 3);
-
FS abundance varied from 0 to 50 CFU/100 ml in June and from 0 to 108 CFU/100 ml in November (Fig. 4).
The results that show the contamination of groundwater affects nearly the whole region (Table 1; Figs. 2, 3, 4). Generally, the highest numbers of these bacteria groups were recorded in June, which is the dry period. One well, however, (no. 17) shows no contamination during the dry period. Otherwise, in the humid period (November) three uncontaminated wells were observed (wells no. 8, 30, and 33). In addition, the abundance of bacteria was lower than in the dry period (June). The highest numbers of bacteria were recorded in Madarh region (Fig. 1). On the other hand, a correlation between pH values and bacteria has been established for the months of June (dry period) and November (humid period): with an increase in pH, the TC and FC values were generally lower (e.g., in the case of wells no. 5, 16, 19, 21, 22, 23, 31 and 34 located in the north of Hassi Smia flexure), whereas the FS values were higher (e.g., in the case of wells no. 15, 29, 30 and 34 located in the south of Hassi Smia flexure). The fecal contamination is due to the injection of fecal organic matter in septic tanks. Therefore, the abundance of bacteria depends on the richness of nutrients from human waste and animals (Al-Barakah F et al. 2017; Bahri and Saibi 2012; Merghem et al. 2016).
Hydrochemistry of groundwater quality
According to Table 1, the pH values of the analyzed samples varied from 7 to 7.64 in March, from 6.7 to 8.9 in June, and from 6.75 to 9.62 in November. These values indicate that the groundwater is slightly acid to alkaline water. The temperature of groundwater ranged between 18.5 and 23.5 °C. The EC values of groundwater samples range from 740 to 5380 µS/cm in March, from 1170 to 4120 µS/cm in June, and from 1590 to 7340 µS/cm in November. The highest EC was recorded in the west part of the study area (Fig. 5; Tables 2, 3).
The hydrogeochemical facies of groundwater and the relationship between different dissolved ions can best be understood by plotting geochemical data on a Piper diagram, which indicates an Na–K–Cl type water (Fig. 6). The diagram shows the evolution of major ions for two periods (March and November). According to the Ca2+, Mg2+, Na+, and K+ triangle, an increase in the concentration of Mg2+ and decreases in Na2+ and K+ were registered.
The results of plots of major ions with EC show that chloride and sodium were strongly correlated with EC with R2 = 0.95 and 0.836, respectively (Fig. 7). These values indicate that the groundwater salinities were mainly controlled by these ions. The possibility that salinization could have been caused by seawater intrusion is not plausible, because Triffa is an unconfined aquifer, with no inflow in its north part. Thus, a plot of chloride versus sodium shows a high correlation coefficient of 0.797 (Fig. 8), indicating that these ions have the same origin: the dissolution of halite present in: (1) sedimentary rocks (DGH 1997; El Mandour 1998; El Idrysy and Smedt 2006) or (2) saline surface deposits (Chettouani and Damou 1993; Benkaddour 1997). The plot of SO4 versus EC (Fig. 7) shows a good correlation, indicating that the sulfate participates in water mineralization. On the other hand, a plot of SO4 versus Ca (Fig. 8) indicates that the sulfate has the same origin as Ca. A plot of HCO3 versus EC (Fig. 7) shows no linear correlation between them and indicates that the bicarbonate participates in the transfer reaction. On the other hand, the plot of HCO3 versus Ca (Fig. 8) indicates the ionic association between them (Saber et al. 2013; Mallick 2017).
The concentrations of NO3 − in March, June, and November are in the range of: 31.6–135.7, 10.5–212.9, and 1.7–168.3 mg/l, those of Cl− are in the range of 329.4–1666.4, 113.6–1480.3, and 159.3–1806.2, and those of SO4 2− are in the range of 163.2–672, 37.5–782.5, and 23.6–672 mg/l, respectively (Fig. 9; Tables 2, 3). The high concentrations result from the presence of cultivation zones, where ammonium nitrate is used as a chemical fertilizer. This observation is similar to those made in other studies carried out in the Triffa plain (Fetouani et al. 2008; Fekkoul et al. 2013). The increases in other anions and cations occur due to the concentration of these ions by recycling the groundwater as irrigation water (Bahri and Saibi 2012). Comparing the concentration of nitrate, chloride, and sulfate with the depth of the aquifer suggests that high concentrations takes place in the dry period. On the other hand, the plots of HCO3 versus SO4 and SO4 versus Cl (Fig. 8) show that the pollutants are inputs from the soil surface and their origin is the same: from fertilizers and from animal and human wastes (Saber et al. 2013; Mallick 2017; Unnisa S and Zainab Bi 2017).
According to WHO standards, the admissible levels of Cu, Zn, Al, Cd, and Fe in potable water are 2, 3, 0.2, 0.003, and 0.2 mg/l, respectively. The concentrations of trace metals in groundwater are generally within the admissible standard range, with the exception of Cu, Zn, Al for wells 24 and 26 measured in June, and wells 1 and 17 measured in November, and Fe for wells 12 and 26 measured in June, and wells 1, 18, 27, and 33 measured in November. For Cd, all samples of groundwater present contamination with a concentration higher than 0.003 mg/l (Table 4). This contamination comes probably from the pesticides used for irrigation and the discharge located in the southern part of Berkane.
PCA
The data were processed by the STATISTICA software package. The variables of Mg, Ca, Na, K, Cl, HCO3, SO4, NO3 and EC were used for the PCA test (Table 5). Three factors were extracted; factor 1 shows strong positive loadings of EC, Mg, Cl, Na and K with 51.91% of Total Variance (TV: 85.45%); factor 2 shows strong negative loadings of NO3, SO4 and Ca with 17.98% of TV; and factor 3 shows a strong negative loading of HCO3 with 15.56 of TV. The spatial distribution of the variables and individuals in the axes system F1-F2 and F1-F3 are showed in Figs. 10 and 11.
Considering the spatial distribution of the variables and individuals in the axe systems of F1-F2 we can conclude the presence of two groups of waters. Group 1 corresponds to the waters from the south-west part of the study area. This group which is located in a fractured zone suggest that the area of recharge (Oued Cherrâa) and the mixture of groundwater between unconfined and confined aquifers facilitated by faults. Group 2 consists of the waters of wells situated in the center of Triffa plain (an intensive irrigation zone), where agricultural activities take place.
Conclusions
The results obtained from the 34 analyzed samples show that groundwater quality in the Triffa aquifer is poor. High concentrations of nitrates and EC were recorded in the western part of the Triffa plain, and in over half of the tested wells exceeded the standard level set for drinking water by the WHO (50 mg/l). The bacteriological data (TC, FC, and FS) showed that almost all of the samples were contaminated. The contamination came from septic tanks and the wastewater dumped in the Charaa wadi (river). In general, the results for metals (Cu, Zn, Al, Fe) were within the acceptable range for drinking water, except for Cd, a contamination that could be dangerous for human life. Multivariate statistical analysis undertaken using PCA of major hydrochemical ions identified three major geochemical processes with 85.45% of TV. Thus, the groundwater from the Triffa plain is recommended for irrigation and domestic use (house cleaning, etc.).
References
AFNOR (1998) Association française de normalisation: Installations classées pour la protection de l’environnement. Oxford University Press, Paris. ISBN: 2-12-214311-8
Al-Barakah FN, Al-jassas AM, Aly AA (2017) Water quality assessment and hydrochemical characterization of Zamzam groundwater, Saudi Arabia. Appl Water Sci. doi:10.1007/s13201-017-0549-x
Baba A (2012) Assessing the impact of climate change on groundwater quality in Turkey. Quatern Int 279–280:31
Bahri F, Saibi H (2012) Characterization, classification, bacteriological, and evaluation of groundwater from 24 wells in six departments of Algeria. Arab J Geosci 5(6):1449–1458
Benkaddour R (1997) Contribution à l’étude de la salinité et de la pollution par les nitrates des eaux souterraines de la plaine des Triffa ‘‘Basse Moulouya’’. Université Mohammed Premier, Oujda
Chettouani B, Damou S (1993) Diagnostic des problèmes d’engorgement des sols, de drainage et de la qualité des eaux et des sols dans la plaine des Triffa (Basse Moulouya). IAV, Mémoire de 3ème cycle en agronomie. Option: génie rural, vol. 1, Rabat
DGH (1997) Etude de l’aquifère superficiel de la plaine de Triffa. Mission I, description et analyse. Rapport définitif. Agence du Bassin Hydraulique de la Moulouya, Oujda, Maroc
Douagui AG, Kouame IK, Koffi K, Goula ATB, Dibi B, Gone DL, Coulibaly K, Seka ANM, Kouassi AK, Oi Mangoua JM, Savane I (2012) Assessment of the bacteriological quality and nitrate pollution risk of quaternary groundwater in the southern part of Abidjan District (Côte d’Ivoire). J Hydroenviron Res 6(3):227–238
El Idrysy E, Smedt F (2006) Modelling groundwater flow of the Trifa aquifer Morocco. Hydrogeol J 14(7):1265–1276
El Idrysy E, Smedt F (2007) A comparative study of hydraulic conductivity estimations using geostatistics. Hydrogeol J 15(3):459–470
El Mandour A (1998) Contribution hydrogéologique de la plaine des Triffa: salinisation et modélisation, Université Mohamed 1er, Faculté des sciences, Oujda
Fekkoul A, Zarhloule Y, Boughriba M, A-e Barkaoui, Jilali A, Bouri S (2013) Impact of anthropogenic activities on the groundwater resources of the unconfined aquifer of Triffa plain (eastern Morocco). Arab J Geosci 6(12):4917–4924
Fetouani S, Sbaa M, Vanclooster M, Bendra B (2008) Assessing ground water quality in the irrigated plain of Triffa (north-east Morocco). Agric Water Manage 95(2):133–142
IPCC (2007) Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jilali A (2014) Impact of climate change on the Figuig aquifer using a numerical model: oasis of eastern Morocco. J Biol Earth Sci 4(1):E16–E24
Jilali A, Abbas M, Amar M, Zarhloule Y (2015a) Groundwater contamination by wastewater in Figuig Oasis (Eastern High Atlas, Morocco). Nat Environ Pollut Technol 14(2):275–282
Jilali A, Fagel N, Amar M, Abbas M, Zarhloule Y (2015b) Hydrogeochemical processes constrained by multivariate statistical methods and isotopic evidence of groundwater recharge in the aquifer of Figuig, Eastern High Atlas of Morocco. Arab J Geosci. doi:10.1007/s12517-015-2089-8
Kumar M, Herbert R Jr, Ramanathan AL, Someshwar Rao M, Kim K, Deka JP, Kumar B (2013) Hydrogeochemical zonation for groundwater management in the area with diversified geological and land-use setup. Chem Erde-Geochem 73(3):267–274
Lamrani Alaoui H, Oufdou K, Mezrioui N (2008) Environmental pollutions impacts on the bacteriological and physicochemical quality of suburban and rural groundwater supplies in Marrakesh area (Morocco). Environ Monit Assess 145(1–3):195–207
Mallick J (2017) Hydrogeochemical characteristics and assessment of water quality in the Al-Saad Lake, Abha Saudi Arabia. Appl Water Sci. doi:10.1007/s13201-017-0553-1
Merghem KA, Jilali A, Alnedhary AA, El Halouani H, Dssouli K (2016) Impact of wastewater on groundwater resources in Sana’a basin, Yemen. Arab J Geosci. doi:10.1007/s12517-015-2281-x
Rodier J (1984) L’analyse de l’eau: eaux naturelles, eaux résiduaires, eau de mer, 7th edn. Dunod Edition, Paris. ISBN: 2-04-015615-1
Saber M, Abdelshafy M, Faragallah M-A, Abd-Alla M (2014) Hydrochemical and bacteriological analyses of groundwater and its suitability for drinking and agricultural uses at Manfalut District, Assuit, Egypt. Arab J Geosci 7(11):4593–4613
Unnisa SA, Zainab Bi S (2017) Groundwater quality characterization around Jawaharnagar open dumpsite, Telangana State. Appl Water Sci. doi:10.1007/s13201-017-0544-2
WHO (2000) Global water supply and sanitation assessment 2000 report. UNICEF, OMS, Geneva
Acknowledgments
The authors wish to thank Dr. Mike Mlynarczyk for helping improve this manuscript and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yahya, H.S.A., Jilali, A., Mostareh, M.M.M. et al. Microbiological, physicochemical, and heavy metals assessment of groundwater quality in the Triffa plain (eastern Morocco). Appl Water Sci 7, 4497–4512 (2017). https://doi.org/10.1007/s13201-017-0598-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0598-1