Abstract
In this study a novel composite desiccant material “CaCl2/Vermiculite/Saw wood” have been synthesized and tested for the water generation from atmospheric air. The vermiculite- saw wood used as a host matrix and CaCl2 as a hygroscopic salt. A solar glass desiccant box type system with a collector area of 0.36 m2 has been used. Design parameters for water generation are height of glass from the desiccant material bed as 0.22 m, inclination in angle as 30º, the effective thickness of glass as 3 mm and number of glazing as single. It has been found that the concentration of calcium chloride is the most influencing factor for fresh water generation from atmospheric air. The maximum amount of water produced by using novel composite desiccant material is 195 ml/kg/day.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a very important relationship between energy and water. Water is required by all the resources of energy in their production, e.g. cooling requirement in thermal processes, cleaning processes, cultivation of bio-fuel crops and power turbine (including electricity), whereas energy is required to make the water resources available to the human being for its daily requirements through transport, treatment, pumping and desalination. Since this is the well-known fact that conventional water resources are depleting day by day and thus strives for the alternate ways for the water production Abualhamayel and Gandhidasan (1997) and Gad et al. (2001) stated that there are three ways to produce water from the atmospheric air as condensation method, absorption–regeneration method and absorption–desorption. The condensation method requires a significant amount of energy for its multiple energy conversion processes. In case of absorption- regeneration method, there is requirement of electricity for the circulation of liquid absorbent by a pump and there are maximum chances of corrosion by the liquid desiccant material. The adsorption-desorption method requires no electricity for its working, operates at low temperature, capacity to utilize the solar energy and of lower cost (Gordeeva et al. 2002). Conducted experiments on integrated desiccant collector system for the generation of water from atmospheric air. A thick corrugated layer of cloth was used as a host matrix and CaCl2 as a desiccant material. The results showed that 1.5 L/m2/day of fresh water can be generated by using this system (Hamed 2003). Conducted experiments for the adsorption of water vapor on a sand layer (matrix) impregnated with CaCl2 (salt). Results showed that rate of absorption depends upon mixing ratio. As the mixing ratio decreases, the adsorption rate decreases (Kabeel 2007). Performed experiments on the glass pyramid solar system to extract water from the atmospheric air. Two beds were prepared by using a cloth and saw wood as a host matrix and saturated with 30 % CaCl2 solution as a salt. Results showed that 2.5 L/day/m2 fresh water can be generated by using this setup (Ji et al. 2007). Used composite adsorbent MCM-41 (Mobile Composite Material) for fresh water production from atmospheric air. The Adsorption capacity of the composite desiccant material was found to be 1.75 kg/kg dry adsorbent. It was stated that new composite material can produce 1.2 kg/day/m2 of fresh water (Hamed et al. 2011). Conducted experiments by using a composite desiccant material for the generation of water from atmospheric air. Sand was used as a bed and CaCl2 as a salt. Results showed that 1.0 L/m2 of fresh water can be generated after regeneration of desiccant material (Kumar and Yadav 2015a). Conducted experiments by using a composite desiccant material, saw wood/CaCl2 for the water generation from atmospheric air. It was found that 180 ml/kg/day of fresh water can be generated by using composite desiccant material (Douglas et al. 2005). Performed experiments for the extraction of water from a gas stream i.e. atmospheric air. The gas stream was adsorbed by a porous adsorbent material having a surface modifying agent. When the condition for saturation of water occurs, the surface modifying agent is desorbed from the surface and the water is collected in a condenser. The apparatus and method of this invention is an advantage because it reduced the size and power consumption for the apparatus (Michel 2013). Discloses a device for extracting water contained in the air by condensation is provided. The device includes: a fan for creating an air flow; a heat transfer fluid evaporator for condensing the water in the air flow created by the fan; and a compressor for compressing the heat transfer fluid evaporated by the evaporator, which compressor is placed in the airflow downstream of the evaporator. A system for producing drinking water from the air is also provided, which includes the aforementioned water extraction device (Kumar and Yadav 2015b). Conducted experiments to investigate the design parameters for solar glass desiccant box type system (SGDBS) for the fresh water generation from atmospheric air. It was stated that for the generation of fresh water the design parameters i.e. height of glass from the desiccant material bed, inclination in angle, effective thickness of glass and number of glazing are 0.22 m, 30º, 3 mm and single respectively. It was also found that 200 ml/kg/day water can be produced by using these design parameters.
In this paper, experimental results for the fresh water production by using composite desiccant material have been presented. Composite desiccant materials have received wide attention because of their excellent property for adsorption/regeneration which could have application in fresh water generation from atmospheric air. The experimental results have shown that new composite desiccant material i.e. CaCl2/Vermiculite/Saw wood has excellent property for adsorption and desorption. Also, the new composite desiccant material has produced 1.08 times more water as compared to the CaCl2/saw wood (Kumar and Yadav 2015).
Experimental study
Sample preparation
The new composite desiccant material has been synthesized by using CaCl2/Vermiculite/Saw wood. The vermiculite and saw wood have been used as a host matrix and CaCl2 as a salt.
The Vermiculite and saw wood consists of flat layers having sufficient space for the accommodation of significant quantities of CaCl2 as shown in Fig. 1a. The host matrix pores with the CaCl2 crystals are shown in Fig. 1b.
For the preparation of the sample-1, half kg of saw wood and half kg of vermiculite is mixed with a solution having 9 % concentration of CaCl2. Three steps have been followed for the preparation of the composite desiccant material: (a) host matrix dehumidification at 80 °C, (b) impregnation of host matrix with solution of CaCl2 at room temperature, and (c) heating in an oven up to 100 °C for 3 h for the removal of water. For the preparation of other five samples, similar method have been adopted with the variation in concentration of CaCl2 with 1 kg mixture of vermiculite and saw wood as shown in Table 1. The concentration of solution for the other sample was 16, 23, 28, 33 and 37 %.
Experimental setup
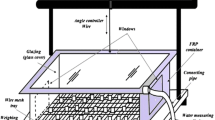
In this experimental study a solar glass desiccant box type system (SGDBS) has been used as shown in Fig. 2a, b. It is fabricated by using fibre reinforced plastic (FRP) for its strength and long life. The dimension of the SGDBS is 0.6 m × 0.6 m × 0.3 m. Two windows of size 0.3 m × 0.3 m have been provided for the process of adsorption. A water collecting tray is provided for the collection of condensed water. A glass of 3 mm thickness has been used as a glazing which also works as a condenser during the regeneration process. A wire mesh, screwed to the plastic frame, having size 3 mm × 3 mm is used for holding the composite desiccant material. For measuring quantity of water produced, measuring cylinder having a minimum capacity of 5 ml is used. The schematic diagram of SGDBS is shown in Fig. 3.
System operation
For the initiation of the experimental setup, the composite desiccant material is placed on the wire mesh tray. For the adsorption process, the side windows of SGDBS are opened at 18:00 h. The adsorption process starts because of the vapor pressure difference between the surface of composite desiccant material and atmospheric air. The composite desiccant material (adsorbent) has lower vapor pressure than that of the atmospheric air. This process is continued till the equilibrium conditions, i.e. when the vapor pressure on the adsorbent surface is the same as that of atmospheric air, attained. In the morning at 7’ o clock, the side windows are closed and the setup is exposed to the sun rays for the regeneration process. As the temperature of the composite desiccant material rises, the vapor pressure difference between the surface of composite desiccant material and the air of the inner space of the box increases. Therefore adsorbed moisture is transferred to the air of the inner space and increases the vapor pressure. As the solar intensity increases, mass transfer of vapor from material to the air of inner space increases and reaches to saturation condition. The water vapor condenses on the inner side of the glass and after coalition forms the small drops. The small drops slide along the surface of the glass and get collected in the water collection tray. Due to slope in the water collection tray the water goes to the water measuring container through a connecting pipe. The maximum regeneration temperature depends upon the available heat. The amount of collected water is measured after regular intervals of 30 min.
Analysis of experimental data
The absorption rate of quantity of desiccant is the amount of water content absorbed by the composite desiccant per unit time and is given by Kumar et al. (2014).
The calculation for the amount of salt required to prepare a solution is given by Kabeel (2007).
and
where, M s is the mass of salt, M w is the mass of distilled water
The instantaneous value of X can be calculated as:
where subscript ‘o’ and ‘i’ are the initial and instantaneous values of X and M sol.
The efficiency for the SGDBS is given by the formula:
Here, M w is the mass of water (kg), L is the latent heat of water at an average bed temperature (J/kg), H is the solar intensity in W/m2, A is the area (m2), \((\tau \alpha )\) is the transmissivity absorbtivity product.
Result and discussion
In this research paper, experiments have been conducted on six samples of composite desiccant materials. The first three samples having 9, 16 and 23 % concentration of CaCl2 in vermiculite/saw wood, have been tested on 9 February, 2015 and other three samples having 28, 33 and 37 % concentration of CaCl2 in vermiculite/saw wood on 10 February, 2015. During the experimental day, maximum solar intensity has been recorded as 947 W/m2 for the first day and 972 W/m2 for the second day.
Case 1: Adsorption rate of composite desiccant material
The experiment for the process of adsorption was initiated at 18:00 hours. Maximum adsorption rate at the initial stage, for sample-1, sample-2, sample-3, sample-4, sample-5 and sample-6 was 0.02637, 0.03062, 0.03864, 0.07704, 0.08672 and 0.1 kg/h respectively. This is because at that time pores were empty and with the progression of time, they started to fill and adsorption rate started to decrease. The maximum adsorption is with sample-6 because it has the maximum concentration of CaCl2 which leads to adsorption of more moisture as compared to the other samples. All samples of composite desiccant materials got saturated at 0:30 h as shown in Figs. 4 and 5.
Case 2: variations in material temperature and solar intensity with time
Figures 6 and 7 show the material temperature distribution of 6 samples of composite desiccant material. It can be seen that the material temperature increase with increase in percentage of CaCl2 at same regeneration condition. This is because when the concentration of CaCl2 is increased then the mass of CaCl2 in host matrix is increased which increases the adsorption capacity for the water content in the sample. Thus the overall capacity for the heat store by the desiccant material increases”.
Case 3: variations in water productivity
The water generation rate depends on the solar radiation intensity, ambient conditions during the day and night, and concentration of the CaCl2 solution in the sample. The adsorption rate of sample 6 is more as compared to the other samples, thus generating maximum amount of water. The maximum generation of water by samples 1, 2, 3, 4, 5, and 6 are 110, 125, 145, 160, 180 and 195 ml/kg/day respectively as shown in Figs. 8 and 9.
Efficiency of the sgdbs
The efficiency of the system can be calculated as:
Result from Fig. 10 shows that the sample having 37 % concentration of CaCl2 have maximum percentage efficiency of SGDBS. This is 1.08 times of sample having 33 % concentration of CaCl2, 1.22 times of sample having 28 % concentration of CaCl2, 1.34 times of sample having 23 % concentration of CaCl2, 1.56 times of sample having 16 % concentration of CaCl2, and 1.77 time of sample having 9 % concentration of CaCl2.
Uncertainity error
The measured values during the experiment are not accurate. These are affected by the deviations caused by various errors.
The errors are calculated for the measuring instruments during the experiments as follows:
% error in measuring intensity = 2 %
% error in measuring temperatures = 0.3 %
% error in measuring water quantity = 0.025 %
Total % error for the SGDBS = 1 × 2 + 4 × 0.3 + 1 × 0.025 = 3.225 %
% of error in weighing machine = 0.1 %
The uncertainty analysis is based on root mean square method as per Kline and McClintock (1953). The relationship for error analysis is given as
where \(f\) is a function of independent variable \(y_{1}\), y 2, etc. stand for the variables of the function \(\Delta y_{1}\), \(\Delta y_{2}\) etc. are the absolute error associated with the variables and \({\raise0.7ex\hbox{${\Delta z}$} \!\mathord{\left/ {\vphantom {{\Delta z} z}}\right.\kern-0pt} \!\lower0.7ex\hbox{$z$}}\) means the relative error. Based on these relationships, the error in efficiency for the experimental setup is ±0.5 %.
Testing of water sample
To check the quality of the water collected from the sample, number of tests have been carried in CSSRI lab, Karnal, Haryana, India on 18, February 2015. The results of chemical and physical tests are shown in Table 2.
Cost analysis of solar glass desiccant based system (SGDBS)
The use of SGDBS depends upon the cost effectiveness. To reduce the dependency on the conventional system for the generation of water, a comprehensive study of cost analysis is presented by Govind and Tiwari (1984).
If P is the initial investment on SGDBS, r % as the annual rate of interest, n as number of useful years to which system will perform and S as salvage value of the SGDBS then,
If annual yield of the system, then
-
(1)
$${\text{Product cost per litre}}\, = \,\frac{\text{Annual cost}}{Y}$$(12)
-
(2)
$${\text{Yield per dollar}} = \frac{\text{Annual yield}}{\text{Annual cost}}$$(13)
For SGDBS
The cost break-up for the SGDBS has been given in Table 3
P = $ 34.05
S = $ 3.66
Assuming n = 15 years, r = 12 % and maintenance cost = 10 % of the total cost,
The cost calculation can be done as follows
CRF = 0.1467
SFF = 0.0268
The final annual cost of the system = CRF × P = 0.1467 × 34.05 = $ 4.99
Annual salvage value = SFF × S = 0.0268 × 3.66 = $ 0.09
Annual maintenance cost = 10 % = 0.10 × 4.99 = $ 0.49
Annual cost = 4.99 + 0.49 = $ 5.48
Annual yield = 195 × 365 = 71,175 ml/kg/year = 71.175 L/kg/year
Generation cost per liter = 5.48/71.175 = $ 0.077
Yield per rupees = 71.175/5.48 = 12.98 L/$
Conclusion
The new composite material for the adsorption of water vapor from atmospheric air has been synthesized by precipitation of calcium chloride in the pores of raw vermiculite/saw wood. Crystalline structure and porous texture of the new composite are studied by SEM technique.
The following conclusion can be drawn as:
-
1.
SEM results of newly composite desiccant material show that the saw wood and vermiculite has flat layers with a distance of 10–40 µm which is enough to accommodate the sufficient quantity of calcium chloride.
-
2.
The results show that maximum adsorption rate is with the sample having 37 % concentration of CaCl2, which is 0.1 kg/h.
-
3.
The results show that maximum generation of water is from the sample having 37 % concentration of CaCl2. This is 1.77 times of sample having 9 % concentration of CaCl2, 1.56 times of sample having 16 % concentration of CaCl2, 1.34 times of sample having 23 % concentration of CaCl2, 1.22 times of sample having 28 % concentration of CaCl2 and 1.08 times of sample having 33 % concentration of CaCl2.
-
4.
The maximum amount of water generated during the experimental days has been reached up to 195 ml/kg/day, which is equal to 500 ml/m2/day from 2.5 kg of composite desiccant material.
-
5.
It has been found from the results that with the increase in the concentration of calcium chloride in vermiculite and saw wood, the water generation rate can be increased.
Abbreviations
- CRF:
-
Capital recovery factor
- G A :
-
Adsorption rate (kg/h)
- H :
-
Solar intensity (W/m2)
- L :
-
Latent heat of water at an average bed temperature (J/kg)
- M sol :
-
Mass of solution
- M s :
-
Mass of salt
- M w :
-
Mass of water
- m ws :
-
Weight of desiccant on wet basis (kg)
- n :
-
Number of useful years
- P :
-
Initial investment
- r %:
-
Annual rate of interest
- S :
-
Salvage value
- SFF:
-
Sinking fund factor
- w :
-
Moisture content in desiccant (kgwater vapor/kgdesiccant)
- X :
-
Concentration of solution
References
Abualhamayel HI, Gandhidasan P (1997) A method of obtaining fresh water from the humid atmosphere. Desalination 113:51–63
Douglas MS, James SD, William L (2005) Production of drinking water from air. U.S. Patent No. 6960243 B1
Gad HE, Hamed AM, El-Sharkawy II (2001) Application of a solar desiccant/collector system for water recovery from atmospheric air. Renew Energ 22:541–556
Gordeeva LG, Restuccia G, Ferni A, Aristov YuI (2002) Water sorption on composites LiBr in a porous carbon. Fuel Process Technol 79:225–231
Govind S, Tiwari GN (1984) Economic analysis of some solar energy systems. Energy Conv Manag 24:131–135
Hamed AM (2003) Experimental investigation on the natural absorption on the surface of sandy layer impregnated with liquid desiccant. Renew Energy 28:1587–1596
Hamed MA, Aly AA, Zeidan BES (2011) Application of solar energy for recovery of water from atmospheric air in climatic zones of Saudi Arabia. Nat Res l2:8–17
Ji JG, Wang RZ, Li LX (2007) New composite absorbent for solar-driven fresh water production from the atmosphere. Desalination 212:176–182
Kabeel AE (2007) Water production from air using multi-shelves solar glass pyramid system. Renew Energy 32:157–172
Kline SJ, McClintock FA (1953) Describing uncertainties in single-sample experiments. Mech Eng 78:3–8
Kumar M, Yadav A (2015a) Experimental investigation of solar powered water production from atmospheric air by using composite desiccant material CaCl2/saw wood. Desalination 367:216–222
Kumar M, Yadav A (2015b) Experimental investigation of design parameters of solar glass desiccant box type system for water production from atmospheric air. J Renew Sustain Energy 7:033122
Kumar A, Chaudhary A, Yadav A (2014) The regeneration of various solid desiccant by using the parabolic dish collector and adsorption rate: an experimental investigation. Int J Green Energy 11:936–953
Michel P (2013) Device for extracting water from the air, and system for the production of drinking water. U.S. patent No. U.S. 20130008196 A1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kumar, M., Yadav, A. Composite desiccant material “CaCl2/Vermiculite/Saw wood”: a new material for fresh water production from atmospheric air. Appl Water Sci 7, 2103–2111 (2017). https://doi.org/10.1007/s13201-016-0406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0406-3