Abstract
The ever-increasing number and production capacity of petroleum refineries in recent years have intensified the need for developing an effective and practical method for treating their wastewaters. In this study, the application of Fenton process with scrap iron powder was investigated for the treatment of a bio-refractory petroleum refinery effluent. Response surface methodology was employed with a cubic IV optimal design to optimize the process using chemical oxygen demand (COD) removal as the target response. H2O2/COD, and H2O2/Fe mass ratios as well as pH were considered as the relevant parameters. A COD removal of more than 83 % was achieved under optimal conditions (H2O2/COD 10.03, H2O2/Fe 2.66 and pH 3.0) within 90 min. Kinetics studies were conducted to investigate the effect of reaction time on COD removal. In addition, the role of post-coagulation on COD removal under optimal conditions was investigated and it was found that 37 % of COD removal occurred due to coagulation, indicating its high potential in the Fenton process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Refining of crude oil is associated with the generation of large amounts of wastewater (0.4–0.6 times the volume of the oil processed) (Coelho et al. 2006). The demand for reduced water consumption in the industry warrants the development of water treatment and water recycle technologies for the oil industry (Saien and Nejati 2007). Petroleum refinery effluents contain large quantities of such toxic and recalcitrant aromatic compounds as Benzene, Toluene, Ethyl benzene, and Xylene which are recognized as the most hazardous compounds released into the environment (Aranda et al. 2010; Tiburtius et al. 2005). The conventional methods used for treating refinery wastewaters include mechanical and physicochemical processes such as oil–water separation, coagulation, and dissolved air flotation with further biological treatment (Stepnowski et al. 2002; Diya’uddeen et al. 2011). However, biological processes are not efficient when the wastewater contains highly toxic and bio-refractory compounds (Saien and Nejati 2007).
Advanced oxidation processes characterized by the generation of hydroxyl radicals (OH·) can be used to destroy a wide range of toxic and bio-refractory compounds. Among these, the Fenton process which utilizes a mixture of hydrogen peroxide and ferrous ions to generate hydroxyl radicals has proved to be an effective and economical method (Eq. 1) (Stepnowski et al. 2002; Chu et al. 2012).
A number of researchers have investigated the Fenton process using alternative iron sources to find that iron powder could be replaced by iron salts as the catalyst in the Fenton reaction. In this case, Fe2+ is generated due to the corrosion of iron in the presence of H2O2 under acidic conditions which would then react with hydrogen peroxide (Barbusiński and Majewski 2003; Su et al. 2011; Chang et al. 2009). Ferric ions in the solution may react with hydrogen peroxide and yield ferrous ions and hydroperoxyl radicals. This reaction is referred as Fenton-like reaction (Hasan et al. 2012). In addition, ferric ions existing in the solution can improve the treatment process by coagulation of organic compounds. The proposed reaction mechanism is shown in Eqs. (2, 3, 4, 5) (Kallel et al. 2009; Benatti et al. 2006; Hasan et al. 2012).
Fenton-like reaction with the constant rate of 0.01–0.02 M−1 s−1 is much slower than Fenton reaction with the constant rate of 76 M−1 s−1, moreover HO ·2 is less reactive than OH·; thus, Fenton is the dominant reaction in production of the radicals (Anotai et al. 2006; Sun et al. 2007; Ramirez et al. 2009). In general, regeneration of ferrous iron from the Fenton-like reaction is the rate limiting step in the iron cycle. As a result, after consumption of ferrous ions by the Fenton reaction, the generation of radicals will considerably slow down (Kavitha and Palanivelu 2004; Anotai et al. 2006).
Industrial wastewater treatment plants deal with problems related to the variability of wastewater characteristics. Therefore, the concentrations of the reagents used should be adjusted to meet optimal treatment conditions (Bianco et al. 2011). Response surface methodology (RSM) is a powerful method to determine optimal operating conditions for processes with a wide variability and different interactions (Ahmadi et al. 2005).
In this study, the Fenton treatment (combination of advanced oxidation and post-coagulation processes) was used with scrap iron powder for the treatment of a bio-refractory petroleum refinery effluent. Response surface methodology with a cubic IV optimal design was used to evaluate and optimize the process. The effects of the parameters involved in the Fenton process including the ratios of hydrogen peroxide to COD and hydrogen peroxide to Fe, pH, and reaction time were also investigated.
Materials and methods
Materials
The effluent from the APIFootnote 1 process was obtained from a plant located in Isfahan Oil Refining Co. in Iran; the process is commonly employed for treating wastewaters from lubrication oil manufacturing plants. Due to the high bio-refractory content of the wastewater, the effluent is transferred into an evaporation pond. The characteristics of the wastewater used in this study are presented in Table 1.
All chemicals (NaOH, HCl, H2SO4, H2O2 30 %) were purchased from Merck (analytical grade). Scrap iron shavings were obtained from a metal tuner. Iron particles were prepared by sieving them and particles ranging between 106 and 300 μm (No. 140–50) in diameters were selected for the experiments. The particles were washed in H2SO4 (0.6 M) for 10 min, and in distilled water before they were washed in NaOH (1 M) for 10 min to remove lipids on the surface of the particles (Mu et al. 2004). Finally, they were washed in distilled water again to remove residual NaOH.
Experimental procedure
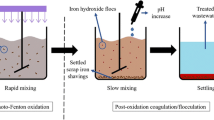
As shown in Fig. 1, the experiments were carried out with samples of 400 mL of wastewater in 1 L containers. NaOH (1 M) and HCl (2 M) were used for pH adjustment. The Fenton process was conducted at room temperature (25 °C) in the batch mode using predetermined concentrations of reagents. A six-blade jar test apparatus was used for agitation (200 rpm) throughout the process. Containers with square cross sections (10 cm × 10 cm) were used to provide complete agitation.
At the end of the reaction time (90 min), oxidation was terminated by adjusting pH to 8.5. This pH value also falls within the optimum range for the coagulation process reported by many researchers (Altaher et al. 2011; Tchobanoglous et al. 2003; Yoon et al. 2002). Hydrogen peroxide breaks down into oxygen and water at pH values above 7.0 (Kallel et al. 2009). It is important to note that each mg/L of residual H2O2 in solution is equivalent to 0.27 mg/L of COD (Ahmadi et al. 2005). Residual H2O2 must, therefore, be allowed to decompose to avoid interference with COD measurement (Anotai et al. 2010; Coelho et al. 2006). The sludge in samples with a high initial H2O2 concentration cannot settle due to the presence of bubbles produced by the decomposition of residual hydrogen peroxide. Hence, 50 mL of each sample was transferred into capped containers and kept at 75 °C for 5 h. After simultaneous precipitation and coagulation of Fe3+ in the form of Fe(OH)3, the supernatant was decanted and subjected to analyses.
Analyses
COD was measured according to the colorimetric method for COD in the range of 0–1,500 mg/L using a HACH DR/2010 spectrophotometer. COD removal efficiency was defined as in the following Equation:
where CODi and CODf designate the measured COD values before and after the Fenton process, respectively.
The pH of wastewater was measured using a 3045 Ion Analyzer pH meter. All other analytical tests were performed according to the standard methods for examination of water and wastewater (Eaton et al. 2005).
Experimental design and optimization
Experiments were carried out in a cubic IV optimal design using the RSM to investigate the effects of the parameters involved. The IV optimal design seeks to minimize the integral of prediction variance across the entire design space. Moreover, it is built algorithmically to provide a lower prediction variance throughout a region of interest (Design Expert software, version 8, user’s Guide 2010).
The mass ratios of H2O2/COD and H2O2/Fe as well as pH were considered as independent variables and COD removal efficiency was considered as the response. Variable levels were determined based on both stoichiometric studies and literature reports to ensure that the design points fell within the design space. The calculation of minimum H2O2 dosage was based on the stoichiometric ratio with respect to complete oxidation of COD as presented in the following Equation (Badawy et al. 2006; Gernjak et al. 2003).
The variables and design levels are presented in Table 2.
The experiment design, optimization, and the 3D response surface plot were accomplished using the Design-Expert software, version 8.0.7.1 (Stat-Ease, Inc., USA). The optimal conditions determined by the software were used for investigating the reaction time and kinetics in the next steps.
Results and discussion
In order to investigate the factors (mass ratios of H2O2/COD, H2O2/Fe and pH) involved in the Fenton reaction, 30 sets of experiments with operating conditions according to the experimental design were performed. All the experiments were conducted during a reaction time of 90 min. A mass-based computation was used for better evaluation of the treatment process. Table 3 presents the experimental results under each operating conditions based on the IV optimal design.
A cubic model was used for design of experiments due to the likelihood of a wavy response and high interactions among the parameters (Design Expert software, version 8, user’s Guide 2010). Analysis of variance was performed to evaluate the interactions among the variables and for model calibration according to experimental results (Table 4).
All the statistically non-significant terms were eliminated from the model. The following equation with coded variables represents COD removal based on the reduced cubic model fitted to the experimental results (Eq. 8).
A, B, and C were already introduced in Table 3.
According to Table 4, the Model F-value of 61.94 implies that the model is significant. There is a chance of only 0.01 % that a “Model F-Value” as large as this would occur due to noise. The “Lack of Fit F-value” of 0.95 implies that the Lack of Fit is not significantly relative to the pure error. The “Adequate Precision” measures the signal to noise ratio. The ratio of 27.824 indicates an adequate signal; thus, this model can be used to navigate the design space.
Optimal treatment conditions
In this study, COD removal was used as an indicator of successful wastewater treatment. In general, the success of the Fenton treatment depends on the generation of OH·. Figure 2 shows the response surface of COD reduction due to the interaction between COD/H2O2 and H2O2/Fe ratios at pH 3.0.
Higher concentrations of H2O2/COD in the presence of iron generated more hydroxyl radicals and enhanced process efficiency. However, excessive amounts of hydrogen peroxide and Fe particles had adverse effects on removal efficiency. The undesirable reactions between hydroxyl/hydroperoxyl radicals and excessive amounts of H2O2 and Fe ions may be explained by the following Eqs. (9, 10, 11, 12).
Although higher concentrations of Fe ions might have been advantageous in terms of COD reduction by post-coagulation, a high dosage of iron ions scavenged the hydroxyl/hydroperoxyl radicals (Benatti et al. 2006; Kang and Hwang 2000).
As the results show (Fig. 3), pH is also a determinant factor in the Fenton process.
The highest concentration of Fe2+ occurred at pH values in the range of 2.0–4.0 and beyond which the efficiency decreased. Moreover, hydrogen peroxide is most stable at pH levels in the range of 3.0–4.0 and its decomposition at higher pH values formed another reason for the inefficiency of the process (Benatti et al. 2006; Badawy et al. 2006). As seen in Fig. 3, maximum COD removal was obtained at a pH value of around 3.0 with COD removal steadily decreasing as pH increased. These results agree well with those reported in the literature (Kallel et al. 2009; Badawy et al. 2006; Kang and Hwang 2000).
The optimal conditions derived from the experiments conducted are presented in Table 5. These values were employed in the investigation of the reaction kinetics and post-coagulation in the Fenton process.
Kinetics studies
Figure 4 shows the variation of COD content versus reaction time after 180 min.
As can be seen, a rapid COD removal was achieved early on so that more than 60 % of COD removal occurred within the first 20 min. While COD reached near its constant value after around 90 min of the reaction time.
The order of reaction was determined by plotting the experimental data functionally, based on the integrated form of the rate expression for a batch reactor. The following expression is for an nth-order reaction carried out in a batch reactor.
where n is the reaction order and k is the reaction rate constant. Integrating the above expression for different reaction orders, between the limits from COD = COD0 to COD = COD and from t = 0 to t = t, results in
where COD represents its value at t = t, COD0 is the initial COD, and k0, k1, and k2 are zero-, first-, and second-order rate constants, respectively (Tchobanoglous et al. 2003).
Figure 5 represents the graphical analysis for determination of reaction order and reaction rate of the process using above equations.
As it can be seen, the linear regression analysis indicates that the second-order reaction model with the slope of k2 = 7 × 10−5 L mg−1 min−1 could be a better describe for the treatment process than the other reaction orders. Similar observations by other researchers have been reported describing the Fenton process by a second-order reaction model (Su et al. 2011; Szpyrkowicz et al. 2001; Leong and Bashah 2012).
Post-coagulation process
In addition to the mineralization of organic compounds due to reaction with hydroxyl radicals discussed above, the Fenton treatment also includes a second stage, i.e. removal by coagulation. While in most of literatures the precipitation of iron ions has just been considered as a stage to remove them from the solution and terminate the oxidation, it can be exploited as a post-coagulation process to enhance the overall treatment efficiency. Hydrolyzed metal ions in the solution can act as coagulants by neutralizing charge of colloidal particles (adsorption of metal ions onto the surface particles) and promoting them to agglomerate, or by bridging between two or more particles, which eventually leads to settling of the flocs; nevertheless, the dominant removal mechanism in the post-coagulation process is enmeshment of colloidal particles by sweeping flocs of metal hydroxide (Tchobanoglous et al. 2003; Altaher et al. 2011). This mechanism may occur due to the precipitation of iron hydroxides by modulating pH to 8.5 to terminate the Fenton reactions. As flocs of metal hydroxide precipitate, the colloidal particles can become entrapped in sweeping flocs and be removed from the wastewater. Efficiency of this process depends on the formation and precipitation of iron hydroxide flocs. The solubility of Fe(OH)3 is a function of pH and the optimum pH range for the sweep floc mechanism is between 7.0 and 9.0 (Tchobanoglous et al. 2003; Altaher et al. 2011; Yoon et al. 2002).
In this study, the post-coagulation process was investigated using 1.7 g/L iron (optimal concentration for the Fenton process) without introduction of H2O2 (the other experimental conditions were the same of those used for the Fenton process). According to Fig. 6, about 37 % of the overall COD removal was due to coagulation, accounting for a high potential for removing organics that allows it to be exploited as an auxiliary process to the advanced oxidation stage.
Therefore, considering the Fenton treatment as the combination of advanced oxidation and post-coagulation processes, and optimizing the other determinant factors in the post-coagulation process such as mixing time and velocity, coagulant dosage, temperature, etc., would make the Fenton treatment a promising method for treating recalcitrant wastewaters.
Conclusion
The results confirmed that the Fenton treatment with scrap iron is an effective method for the pre-treatment of petroleum refinery wastewaters containing high concentrations of recalcitrant compounds. Over 83 % of the COD was removed within 90 min at pH 3.0 and at mass ratios of 10.03 and 2.66 for H2O2/COD and H2O2/Fe, respectively. Compared to iron salts, scrap iron used in the Fenton treatment has a twofold advantage: possibility of multiple use and low costs. The post-coagulation process after Fenton oxidation is an advantageous stage and should be investigated and optimized individually to enhance overall treatment efficiency.
Notes
- American Petroleum Institute
References
Ahmadi M, Vahabzadeh F, Bonakdarpour B, Mofarrah E, Mehranian M (2005) Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J Hazard Mater 123(1–3):187–195. doi:10.1016/j.jhazmat.2005.03.042
Altaher H, ElQada E, Omar W (2011) Pretreatment of wastewater streams from petroleum/petrochemical industries using coagulation. Adv Chem Eng Sci 1:245–251. doi:10.4236/aces.2011.14035
Anotai J, Lu M-C, Chewpreecha P (2006) Kinetics of aniline degradation by Fenton and electro-Fenton processes. Water Res 40(9):1841–1847. http://dx.doi.org/10.1016/j.watres.2006.02.033
Anotai J, Su C-C, Tsai Y-C, Lu M-C (2010) Effect of hydrogen peroxide on aniline oxidation by electro-Fenton and fluidized-bed Fenton processes. J Hazard Mater 183(1–3):888–893. doi:10.1016/j.jhazmat.2010.07.112
Aranda E, Marco-Urrea E, Caminal G, Arias ME, García-Romera I, Guillén F (2010) Advanced oxidation of benzene, toluene, ethylbenzene and xylene isomers (BTEX) by Trametes versicolor. J Hazard Mater 181(1–3): 181–186. doi:http://dx.doi.org/10.1016/j.jhazmat.2010.04.114
Badawy MI, Ghaly MY, Gad-Allah TA (2006) Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 194(1–3):166–175. doi:10.1016/j.desal.2005.09.027
Barbusiński K, Majewski J (2003) Discoloration of azo dye Acid Red 18 by Fenton reagent in the presence of iron powder. Pol J Environ Stud 12(2):151–155. http://www.pjoes.com/pdf/12.2/151-155.pdf
Benatti CT, Tavares CRG, Guedes TA (2006) Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology. J Environ Manage 80(1):66–74. doi:10.1016/j.jenvman.2005.08.014
Bianco B, De Michelis I, Vegliò F (2011) Fenton treatment of complex industrial wastewater: optimization of process conditions by surface response method. J Hazard Mater 186(2–3):1733–1738. doi:10.1016/j.jhazmat.2010.12.054
Chang S-H, Chuang S-H, Li H-C, Liang H-H, Huang L-C (2009) Comparative study on the degradation of I.C. Remazol Brilliant Blue R and I.C. Acid Black 1 by Fenton oxidation and Fe0/air process and toxicity evaluation. J Hazard Mater 166(2–3):1279–1288. doi:10.1016/j.jhazmat.2008.12.042
Chu L, Wang J, Dong J, Liu H, Sun X (2012) Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere 86(4):409–414. doi:10.1016/j.chemosphere.2011.09.007
Coelho A, Castro AV, Dezotti M, Sant’Anna GL Jr (2006) Treatment of petroleum refinery sour water by advanced oxidation processes. J Hazard Mater 137(1):178–184. doi:10.1016/j.jhazmat.2006.01.051
Design Expert software, version 8, user’s Guide (2010). Stat-Ease, USA
Diya’uddeen BH, Daud WMAW, Abdul Aziz AR (2011) Treatment technologies for petroleum refinery effluents: a review. Process Saf Environ 89(2):95–105. doi:10.1016/j.psep.2010.11.003
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (2005) Standard methods for the examination of water and wastewater. Centennial edition, 21st edn. American Public Health Association, Washington, DC
Gernjak W, Krutzler T, Glaser A, Malato S, Caceres J, Bauer R, Fernández-Alba AR (2003) Photo-Fenton treatment of water containing natural phenolic pollutants. Chemosphere 50(1): 71–78. http://dx.doi.org/10.1016/S0045-6535(02)00403-4
Hasan DUB, Abdul Aziz AR, Daud WMAW (2012) Oxidative mineralisation of petroleum refinery effluent using Fenton-like process. Chem Eng Res Des 90(2):298–307. doi:10.1016/j.cherd.2011.06.010
Kallel M, Belaid C, Mechichi T, Ksibi M, Elleuch B (2009) Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem Eng J 150(2–3):391–395. doi:10.1016/j.cej.2009.01.017
Kang YW, Hwang K-Y (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34(10):2786–2790. doi:10.1016/s0043-1354(99)00388-7
Kavitha V, Palanivelu K (2004) The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 55(9):1235–1243. doi:10.1016/j.chemosphere.2003.12.022
Leong SK, Bashah NAA (2012) Kinetic study on COD removal of palm oil refinery effluent by UV-Fenton. APCBEE Procedia 3:6–10. doi:10.1016/j.apcbee.2012.06.037
Mu Y, Yu H-Q, Zheng J-C, Zhang S-J, Sheng G-P (2004) Reductive degradation of nitrobenzene in aqueous solution by zero-valent iron. Chemosphere 54(7):789–794. doi:10.1016/j.chemosphere.2003.10.023
Ramirez JH, Duarte FM, Martins FG, Costa CA, Madeira LM (2009) Modelling of the synthetic dye Orange II degradation using Fenton’s reagent: From batch to continuous reactor operation. Chem Eng J 148 (2–3): 394–404. http://dx.doi.org/10.1016/j.cej.2008.09.012
Saien J, Nejati H (2007) Enhanced photocatalytic degradation of pollutants in petroleum refinery wastewater under mild conditions. J Hazard Mater 148(1–2):491–495. doi:10.1016/j.jhazmat.2007.03.001
Stepnowski P, Siedlecka EM, Behrend P, Jastorff B (2002) Enhanced photo-degradation of contaminants in petroleum refinery wastewater. Water Res 36(9):2167–2172. doi:10.1016/s0043-1354(01)00450-x
Su C-C, Pukdee-Asa M, Ratanatamskul C, Lu M-C (2011) Effect of operating parameters on decolorization and COD removal of three reactive dyes by Fenton’s reagent using fluidized-bed reactor. Desalination 278(1–3):211–218. doi:10.1016/j.desal.2011.05.022
Sun J-H, Sun S-P, Fan M-H, Guo H-Q, Qiao L-P, Sun R-X (2007) A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J Hazard Mater 148(1–2): 172–177. http://dx.doi.org/10.1016/j.jhazmat.2007.02.022
Szpyrkowicz L, Juzzolino C, Kaul SN (2001) A Comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and fenton reagent. Water Res 35(9):2129–2136. doi:10.1016/s0043-1354(00)00487-5
Tchobanoglous G, Burton F, Stensel HD (2003) Metcalf & Eddy, wastewater engineering, treatment, disposal & reuse, 4th edn. McGraw-Hill, New York
Tiburtius ERL, Peralta-Zamora P, Emmel A (2005) Treatment of gasoline-contaminated waters by advanced oxidation processes. J Hazard Mater 126(1–3):86–90. doi:10.1016/j.jhazmat.2005.06.003
Yoon J, Kim Y, Huh J, Lee Y, Lee D (2002) Roles of oxidation and coagulation in Fenton process for the removal of organics in landfill leachate. J Ind Eng Chem 8(5):410–418. http://www.cheric.org/PDF/JIEC/IE08/IE08-5-0410.pdf
Acknowledgments
The authors would like to thank the National Iranian Oil Refining & Distribution Company for their financial support. Thanks also go to Dr. Ezzatollah Roustazadeh from ELC of IUT for his editing of the first English draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Saber, A., Hasheminejad, H., Taebi, A. et al. Optimization of Fenton-based treatment of petroleum refinery wastewater with scrap iron using response surface methodology. Appl Water Sci 4, 283–290 (2014). https://doi.org/10.1007/s13201-013-0144-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0144-8