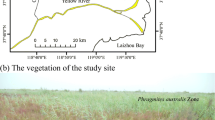
Abstract
Effects of salinity, plant competition and shading on Phragmites australis and Spartina alterniflora were evaluated in greenhouse incubations of intact wetland sediments collected from natural plant stands. Salinity and competition had significant negative effects on growth of both species. Competition reduced growth of both species, but at higher salinity there was a disproportionately large reduction in P. australis growth compared to S. alterniflora. The result was observed in comparisons of both above ground biomass and underground recoverable reserves. The greater negative effect on growth of P. australis appears to result from its lower tolerance than S. alterniflora, to salinities typical of non-riverine New England salt marshes. Increased shading reduced growth of S. alterniflora at higher salinities, suggesting an additional factor potentially influencing its competition with P. australis. Findings agree with previous studies’ suggestions of the importance of interactions between stress tolerance and competition in determining wetlands plant species zonation. Enhancing competition with P. australis may provide another approach for Phragmites control. While current methods typically focus on removal of colonizing stands, parallel introduction of native species, such as S. alterniflora might also be considered, either via favorable habitat creation or through direct transplants, as a potentially means of faster replacement.





Similar content being viewed by others
References
Aphalo PJ, Ballare CL, Scopel AL (1999) Plant-plant signaling, the shade-avoidance response and competition. Journal of Experimental Botany 50(340):1629–1634
Bart D, Hartman JM (2000) Environmental determinants of Phragmites australis in a New Jersey salt marsh: and experimental approach. Oikos 89:59–69
Bart D, Burdick D, Chambers R, Hartman JM (2006) Human facilitation of Phragmites australis invasions in tidal marshes: a review and synthesis. Wetlands Ecology and Management 14:53–65
Bazzaz FA (1979) The physiological ecology of plant succession. Annual Review of Ecology, Evolution, and Systematics 10:351–371
Berry EW (1914) Upper cretaceous and eocene floras of South Carolina and Georgia. U.S. Geological Survey Professional Paper No. 84
Bertness MD (1991a) Zonation of Spartina spp. in New England salt marshes. Ecology 72:138–148
Bertness MD (1991b) Interspecific interactions among high marsh perennials. Ecology 72:125–137
Blanch SJ, Ganf GG, Walker KF (1999) Tolerance of riverine plants to flooding and exposure indicated by water regime. Regulated Rivers 15:43–62
Burdick DM, Konisky RA (2003) Determinants of expansion for Phragmites australis, common reed, in natural and impacted coastal marshes. Estuaries 26:407–416
Buttery BR, Lambert JM (1965) Competition between Glyceria Maxima and Phragmites Communis in the region of Surlingham Broad: I. The competition mechanism. Journal of Ecology 53(1):163–181
Chambers RM, Mozdozer TJ, Ambrose JC (1998) Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal salt marsh. Aquatic Botany 62:161–169
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany 64:261–273
Chambers RM, Osgood DT, Bart DJ, Montalto F (2003) Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26(2B):309–406
Chapman VJ (1974) Salt marshes and salt deserts of the world. Ecology of Halophytes. Academic, New York, pp 3–19
Clark JS (1986) Late-holocene vegetation and coastal processes at a long island tidal marsh. Journal of Ecology 74:561–578
Cross DH and Fleming KL (1989) Control of Phragmites or common reed. USFWS Leaflet 13.4.12
Emery NC, Ewanchuck PJ, Bertness MD (2001) Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology 82(9):2471–2485
Engloner AI (2007) Structure, growth dynamics and biomass of reed (Phragmites australis)—A review. Flora 204:331–347
Gallagher JL (1983) Seasonal patterns in recoverable underground reserves in Spartina alterniflora Loisel. American Journal of Botany 70(2):212–215
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Australian Journal of Plant Physiology 15:63–92
Greiner La Peyre MK, Grace JB, Hahn E, Mendelssohn IA (2001) The importance of competition in regulating plant species abundance along a salinity gradient. Ecology 82(1):62–69
Gusewell S, Edwards P (1999) Shading by Phragmites australis: a threat for species-rich fen meadows? Applied Vegetation Science 2:61–70
Haslam SM (1971) Community regulation in Phragmites Communis Trin: I Monodominant stands. Journal of Ecology 59(1):75–88
Hellings SE, Gallagher JL (1992) The effects of salinity and flooding on Phragmites australis. Journal of Applied Ecology 29(1):41–49
Howes BL, Dacey JWH, Wakeham SG (1985) Effects of sampling technique on measurements of porewater constituents in salt marsh sediments. Limnology and Oceanography 30:221–227
Howes BL, Dacey JWH, Goehringer DD (1986) Factors controlling the growth form of Spartina alterniflora: feedbacks between above-ground production, sediment oxidation, nitrogen and salinity. Journal of Ecology 74:881–898
Howes BL, Goehringer DD (1994) Porewater drainage and dissolved organic carbon and nutrient losses through the intertidal creekbanks of a New England salt marsh. Marine Ecology Progress Series 114:289–301
Howes BL, Teal JM, Peterson S (2005) Experimental Phragmites control through enhanced sediment sulfur cycling. Ecological Engineering 25(3):292–303
Jerling L, Liljelund LE (1984) Dynamics of Plantago maritime along a distributional gradient: a demographic study. Holartic Ecology 7:280–288
Kirk H, Paul J, Straka J, Freeland JR (2011) Long-distance dispersal and high genetic diversity are implicated in the invasive spread of the common reed, Phragmites australis (Poaceae), in northeastern North America. American Journal of Botany 98(7):1180–1190
Lamotte RS (1952) Catalogue of Cenozoic plants of North America through 1950. Geological Society of America. Memoir 51
Lissner J, Schierup H (1997) Effects of salinity on the growth of Phragmites australis. Aquatic Botany 55:247–260
Maestre FT, Valladares F, Reynolds JF (2006) The stress-gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: further insights from arid environments. Journal of Ecology 94:17–22
Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009) Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology 97:199–205
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S (2000) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecology and Management 8:89–103
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecological Applications 13(5):1400–1416
Minchinton TE, Simpson JC, Bertness MD (2006) Mechanisms of exclusion of native coastal marsh plants by an invasive grass. Journal of Ecology 94(2):342–354
Mitsch WJ, Gosselink JG (2000) Wetlands. Wiley, New York
Orson RA, Warren RS, Niering WA (1987) Development of a tidal marsh in a New England river valley. Estuaries 10:20–27
Pearcy RW, Ustin SL (1984) Effects of salinity on growth and photosynthesis of three California tidal marsh species. Oecologia 62:68–73
Pennings SC, Grant M, Bertness MD (2005) Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and competition. Journal of Ecology 93:159–167
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed Phragmites australis into North America. Proceedings of the National Academy of Sciences of the United States of America 99(4):2445–2449
Scheiner D (1976) Determination of ammonia and kjeldahl nitrogen by indophenol method. Water Research 10:31–36
Silvestri S, Defina S, Marani M (2005) Tidal regime, salinity and salt marsh plant zonation. Estuarine, Coastal and Shelf Science 62(1–2):119–130
Taylor N (1939) Salt tolerance of Long Island salt marsh plants. Circular New York State Museum 23:1–42
Teal JM, Peterson S (2005) The interaction between science and policy in the control of Phragmites in olgiohaline marshes of Delaware Bay. Restoration Ecology 13(1):223–227
Tilman D (1982) Resource competition and community structure. Monographs Population Biology 17. Princeton University Press, Princeton, N.J. 296
van Der Toorn J, Mook JH (1982) The influence of environmental factors and management on stands of Phragmites australis. I. Effects of burning, frost and insect damage on shoot density and shoot size. Journal of Applied Ecology 19(2):477–499
Vasquez EA, Glenn EP, Gunternspergen GR, Brown JJ, Nelson SG (2006) Salt tolerance and osmotic adjustment of Spartina alterniflora (poaceae) and the invasive M haplotype of Phragmites australis (poaceae) along a salinity gradient. American Journal of Botany 93(12):1784–1790
Acknowledgments
The authors would like to thank Dr. John M. Teal for insights into the conduct of this research and for careful review of this manuscript and technical staff of the Coastal Systems Program, J. Benson and S. Horvet for assistance in the conduct of the greenhouse experiments and C. David for field assistance. This research was funded through the Janet N. Phiphard Endowment at the University of Massachusetts Dartmouth Foundation, the Sesuit Creek Restoration Project (NOAA/Town of Dennis, MA) and the Coastal Systems Program, SMAST-UMASS Dartmouth.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medeiros, D.L., White, D.S. & Howes, B.L. Replacement of Phragmites australis by Spartina alterniflora: The Role of Competition and Salinity . Wetlands 33, 421–430 (2013). https://doi.org/10.1007/s13157-013-0400-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0400-6