Abstract
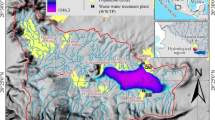
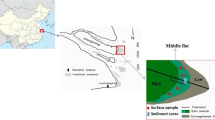
The Gavkhuni playa lake consists of sand, mud, and salt flats. The salt pan covers extensive part of the playa. Its color is usually clear and white, but black, pink and green colors also occur. The black color of halite has been caused by impurities of detrital sediments. The sand detrital sediments have been derived from the aeolian sands located in the west of the playa lake. The pink to light red color of the halite is due to the existence of iron oxides or/and microbial effects. There are potentials for natural concentrations of heavy metals in the evaporite sediments of this lake especially due to the occurrence of sedimentary Pb/Zn ore deposits in its drainage basin. To study the concentration of the heavy metals in the salt pan, 18 samples were taken from the salt pan and analyzed. The results show that average concentrations (ppm) of the heavy metals in the salt pan are Ni (56.46), Sr (26.46), Pb (11.42), Ag (10.70), Mn (6.15), Co (2.86), Cd (1.98), Zn (1.48) and Cu (1.14) in their order abundances. The amounts of Zn, Mn, Sr, Cu, Cd and Pb are relatively high in samples containing calcium minerals. The concentrations of Mn and Cu in the pink and green salts are relatively higher than the white ones, because these metals tend to be adsorbed by organic matter. Manganese oxides are important factors influencing the Ni concentration in the sediments. The Mn and Sr concentrations increase in the samples containing iron silicate minerals and carbonate grains. The Co and Ni concentrations are high in the samples containing Fe/Mg-bearing clastic grains. The Ag concentration is high in the samples containing sulfide minerals. Strong adsorption of Mn2+, Co2+ and Zn2+ to clay minerals and precipitation of Cu as Cu°, Cu2S, CuS and Pb as PbCO3 and PbS in the mud sediments can be the reasons for the lower concentrations of these elements in the pure salt sediments than the mud deposits. Enrichment factor indicates that Ag is moderately enriched and other elements are weakly enriched in the evaporite deposits.




Similar content being viewed by others
References
Acosta JA, Jansen B, Kalbitz K, Faz A, Martínez-Martínez S (2011) Salinity increases mobility of heavy metals in soils. Chemosphere. 85:1318–1324
Adriano DC (2001) Trace elements in terrestrial environments. Springer, Berlin
Alavi M (1994) Tectonics of the Zagros orogenic belt of Iran: new data and interpretations. Tectonophysics 229:211–238
Alloway BJ (1994) Heavy metals in soils. Chapman and Hall, London
Alpers CN, Blowes DW, Nordstrom DK, Jambor JL (1994) Secondary minerals and acid mine-water chemistry. In: Jambor JL, Blowes, DW (eds). Short course handbook on environmental geochemistry of sulfide mine-wastes. Mineral Assoc Can, pp 247–70
Amdouni R (2009) Behaviour of trace elements during the natural evaporation of sea water: case of solar salt works of sfax saline (S.E of Tunisia). Glob NEST J 11(1):96–105
Bjorklund AJ (1983) Geochemical exploration: selected papers from the tenth international geochemical exploration symposium-third symposium on methods of geochemical prospecting. Elsevier, Amsterdam
Bradl HB (2005) Sources and origins of heavy metals, heavy metals in the environment, Elsevier Academic Press, Amesterdam
Brookins DG (1988) Eh-pH diagrams for geochemistry. Springer, Berlin
Buckby T, Black S, Coleman ML, Hodson ME (2003) Fe-sulphate-rich evaporative mineral precipitates from the Rio Tinto, southwest Spain. Miner Mag 67:263–278
Carmona DM, Faz A, Arocena JM (2009) Cadmium, copper, lead, and zinc in secondary sulfate minerals in soils of mined areas in Southeast Spain. Geoderma 150:150–157
Duzzin B, Pavoni B, Donazzolo R (1988) Macroinvertebrate communities and sediments as pollution indicators for heavy metals in the River Adige (Italy. Water Res 22:1353–1363
Eugster HP, Hardie LA (1978) Saline lakes. In: Lakes: Chemistry, Geology and Physics, Springer, Berlin
Frau F (2000) The formation-dissolution-precipitation cycle of melanterite at the abandoned pyrite mine of Genna Luas in Sardinia, Italy: environmental implications. Mineral Mag 64:995–1006
Furquim SAC, Graham RC, Barbiero L, Queiroz Neto JP, Vallès V (2008) Mineralogy and genesis of smectites in an alkaline–saline environment of Pantanal wetland, Brazil. Clays Clay Miner 56:580–596
Gambrell RP, Wiesepape JB, Patrick WH, Duff MC (1991) The effects of pH, redox and salinity on metal release from a contaminated sediment. Water Air Soil Pollut 57:359–367
Goldsmith SL, Krom MD, Sandler A, Herut B (2001) Spatial trends in the chemical composition of sediments on the continental shelf and slope off the Mediterranean coast of Israel. Cont Shelf Res 21(16):1879–1900
Gomes EP, Favas P (2006) Mineralogical controls on mine drainage of the abandoned Ervedosa tin mine in north-eastern Portugal. Appl Geochem 21:1322–1334
Hamdi-Aissa B, Valles V, Aventurier A, Ribolzi O (2004) Soils and brine geochemistry and mineralogy of hyperarid desert playa, Ouargla Basin, Algerian Sahara. Arid Land Res Manag 18:103–126
Handford CR (1981) A process-sedimentary framework for characterizing recent and ancient sabkhas. Sediment. Geol. 30:255–265
Hardie IA, Smoot JP, Eugster HP (1978) Saline lakes and their deposits: a sedimentological approach. In: Matter A, Tucker ME (eds) Modern and Ancient Lake Sediments. Blackwell, London, p 290
Hatje V, Payne TE, Hill DM, McOrist G, Birch GF, Szymczak R (2003) Kinetics of trace element uptake and release by particles in estuarine waters: effects of pH, salinity, and particle loading. Environ Int 29:619–629
Hovorka S (1987) Depositional environment of marine-dominated bedded halite, Permian San Andres Formation, Texas. Sedimentology 34:1029–1054
Humphries MS, Kindness A, Ellery WN, Hughes JC (2010) Sediment geochemistry, mineral precipitation and clay neoformation on the Mkuze River floodplain, South Africa. Geoderma 157(1–2):325–334
Jalali M, Khanlari ZV (2006) Mobility and distribution of Zinc, Cadmium and Lead in calcareous soils receiving spiked sewage sludge. Soil Sediment Contam 15(6):603–620
Jenne EA (1968) Controls on Mn, Fe Co, Ni, Cu and Zn concentrations in soils and water: the significant role of hydrous Mn and Fe oxides. Adv Chemic 73:337–387
Kendall AC (1984) Evaporites. In: Walker RG (ed) Facies Models. Geoscience, Canada, pp 259–296
Last W (1989) Sedimentology of a saline playa in the northern Great Plains, Canada. Sedimentology 36(1):109–123
Lores EM, Pennock JR (1998) The effect of salinity on binding of Cd, Cr, Cu and Zn to dissolved organic matter. Chemosphere 37:861–874
Marengo E, Gennaro MC, Robotti E, Rossanigo P, Rinaudo C, Roz-Gastaldi M (2006) Investigation of anthropic effects connected with metal ions concentration, organic matter and grain size in Bormida river sediments. Anal Chim Acta 560:172–183
Martincic D, Kwokal Z, Branica M (1990) Distribution of zinc, cadmium and copper between different size fraction of sediments the Krka River estuary and the Kornati Islands (Central Adriatic Sea). Sci Total Env 95:217–225
Mason B (1966) Principles of Geochemistry. Wiley, New York
McBride MB (1994) Environmental chemistry of soils. Oxford University Press Inc, New York
Micό C, Recatala L, Peris M, Sanches J (2008) Discrimination of lithogenic and anthropogenic metals in calcareous agricultural soils. Soil Sediment Contam. 17:467–485
Morillo J, Usen J, Gracia I (2007) Potential mobility of metals in polluted coastal sediments in two bays of southern Spain. J Coast Res 23(2):352–361
Paalman MAA, van der Weijden CH, Loch JPG (1994) Sorption of cadmium on suspended matter under estuarine conditions: competition and complexation with major seawater ions. Water Air Soil Pollut 73:49–60
Pakzad HR (2003) Sedimentary facies association of the lower reaches of the Zayandehrud River and the Gavkhoni playa lake basin, Esfahan province, Iran: Doctoral dissertation, Clausthal University
Pakzad HR, Fayazi F (2007) Sedimentology and stratigraphic sequence of the Gavkhoni playa lake, SE Esfahan, Iran. Carbonates Evaporites 22(2):93–100
Pakzad HR, Makizadeh MA, Pasandi M, Aliniaei Z (2012) Composition and origin of aeolian and fluvial sands of Gavkhuni playa lake (southeast of Isfahan, Iran). Stratigr Sedimentol Res 28(3):65–82 (in persian)
Pedron F, Petruzzelli G, Barbafieri M, Tassi E (2009) Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere 75:808–814
Rahimi H, Pakzad HR, Pasandi M (2012) Study of total and exchangeable concentrations of Cu, Ag, Sr, Ni and Mn in mud flat of Gavkhoni playa lake (South east of Isfahan, Iran). Stratigr Sedimentol Res 28(1):65–96 (in persian)
Soylak M, Peker DSK, Turkoglu O (2008) Heavy metal contents of refined and unrefined table salts from Turkey, Egypt and Greece. Environ Monit Assess 143:267–272
Storer DA (1988) A simple high volume ashing procedure for determining soil organic matter. Commun Soil Sci Plant Anal 15:759–772
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu Hawaii. Environ Geol 39:611–637
Tanji KK, Dahlgren RA (1994) Accumulation of toxic trace elements in evaporites in agricultural evaporation ponds: University of California Water Resources Center, 84 p
Teimuri-Asl F, Pakzad HR, Bagheri H (2011) Research on ore forming metals and fluids in Irankuh Pb, Zn ore deposit. J stratigr sediment Res 27(3):83–102 (in Persian)
The Non-Affiliated Soil Analysis Work Committee (1990) Handbook of standard soil testing methods for advisory purposes. Soil Science Society of South Africa, Pretoria, Sunnyside 0132
Tsai LJ, Yu KC, Chang JS, Ho SH (1998) Fractionation of Heavy Metals in Sediment Cores from the Ell-Ren River, Taiwan. Water Sci Technol 37(6–7):217–224
Usman MA, Filli KB (2011) Determination of essential elements and heavy metals contained in table salt. J Res Natl.Dev 9(2):73–78
Valente TM, Leal Gomes C (2009) Occurrence, properties and pollution potential of environmental minerals in acid mine drainage. Sci Total Environ 407:1135–1152
Watson A (1989) Desert Crusts and Rock Varnish. In: Thomas DSG (ed) Arid Zone Geomorphology. Belhaven Press, London
Wedpohl KH (1972) Handbook of Geochemistry. Springer, Berlin
Acknowledgments
Financial support by University of Isfahan is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pakzad, H.R., Pasandi, M. & Zaheri, M. Heavy metal distribution in the salt pan of Gavkhuni playa lake (southeast of Isfahan, Iran). Carbonates Evaporites 30, 135–143 (2015). https://doi.org/10.1007/s13146-014-0187-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-014-0187-4