Abstract
Cycliophorans are characterized by a complex life cycle that involves an asexual generation and a sexual generation. The most prominent life cycle stage in both generations is the so-called feeding stage. Using RNA-Seq, we profiled differential gene expression between feeding stages from asexual and sexual generations to study this intergenerational shift. For this, we also generated a reference transcriptome for the cycliophoran Symbion pandora. We found that a total of 2660 contigs (more than 10% of the total transcriptome) correspond to genes that are expressed differentially in the feeding stages from the asexual generation as compared to the sexual feeding stages. Among these, 1236 genes are upregulated in the asexual stages as compared to the sexual stages. Conversely, 1424 genes are upregulated in the sexual stages as compared to the asexual stages. The asexual stages express genes predominantly related to RNA processing and splicing as well as protein folding, which suggests a high degree of regulation at the transcriptional and post-transcriptional levels. In marked contrast, the sexual stages highly express genes related to signal transduction and neurotransmission. This is the first time that a large whole-transcriptome RNA-Seq expression dataset has been generated for any cycliophoran. Moreover, this study provides important information for further studies on the molecular mechanisms that are involved in the shift from asexual to sexual generations in this still enigmatic group.






Similar content being viewed by others
References
Anders, S., Pyl, P. T., & Huber, W. (2015). HTSeq—a python framework to work with high-throughput sequencing data. Bioinformatics, 31(2), 166–169.
Baker, J. M., & Giribet, G. (2007). A molecular phylogenetic approach to the phylum Cycliophora provides further evidence for cryptic speciation in Symbion americanus. Zoologica Scripta, 36, 353–359.
Baker, J. M., Funch, P., & Giribet, G. (2007). Cryptic speciation in the recently discovered American cycliophoran Symbion americanus; genetic structure and population expansion. Marine Biology, 151, 2183–2193.
Castro, M. (1992). A methodology for obtaining information on the age structure and growth rates of the Norway lobster, Nephrops norvegicus (L.) (Decapoda, Nephropoidea). Crustaceana, 63, 29–43.
Funch, P. (1996). The chordoid larva of Symbion pandora (Cycliophora) is a modified trochophore. Journal of Morphology, 230, 231–263.
Funch, P., & Kristensen, R. M. (1995). Cycliophora is a new phylum with affinities to Entoprocta and Ectoprocta. Nature, 378, 711–714.
Funch, P., & Kristensen, R. M. (1997). Cycliophora. In F. W. Harrison & R. M. Woollacott (Eds.), Microscopic anatomy of invertebrates, lophophorates, entoprocta and cycliophora (Vol. 13, pp. 409–474). New York: Wiley-Liss.
Funch, P., & Kristensen, R. M. (1999). Cycliophora. In E. Knobil & J. D. Neill (Eds.), Encyclopaedia of reproduction (Vol. 1, pp. 800–808). New York: Academic.
Funch, P., Thor, P., & Obst, M. (2008). Symbiotic relations and feeding biology of Symbion pandora (Cycliophora) and Triticella flava (Bryozoa). Vie et Milieu, 58, 185–188.
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Full-length transcriptome assembly from RNA-seq data without a reference genome. Nature Biotechnology, 29, 644–652.
Langmead, B., & Salzberg, S. (2012). Fast gapped-read alignment with Bowtie 2. Nature Methods, 9, 357–359.
Love, M. I., Huber, W., & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15(12), 550.
Neves, R. C., & Reichert, H. (2015). Microanatomy and development of the dwarf male of Symbion pandora (phylum Cycliophora): new insights from ultrastructural investigation based on serial section electron microscopy. PloS One, 10, e0122364.
Neves, R. C., Cunha, M. R., Kristensen, R. M., & Wanninger, A. (2010a). Comparative myoanatomy of cycliophoran life cycle stages. Journal of Morphology, 271, 596–611.
Neves, R. C., Cunha, M. R., Funch, P., Wanninger, A., & Kristensen, R. M. (2010b). External morphology of the cycliophoran dwarf male: a comparative study of Symbion pandora and S. americanus. Helgoland Marine Research, 64, 257–262.
Neves, R. C., Kristensen, R. M., & Funch, P. (2012). Ultrastructure and morphology of the cycliophoran female. Journal of Morphology, 273, 850–869.
Neves, R. C., Bailly, X., & Reichert, H. (2014). Are copepods secondary hosts of Cycliophora? Organisms Diversity & Evolution, 14, 363–367.
Obst, M., & Funch, P. (2003). Dwarf male of Symbion pandora (Cycliophora). Journal of Morphology, 255, 261–278.
Obst, M., & Funch, P. (2006). The microhabitat of Symbion pandora (Cycliophora) on the mouthparts of its host Nephrops norvegicus (Decapoda: Nephropidae). Marine Biology, 148, 945–951.
Obst, M., Funch P., & Giribet G. (2005). Hidden diversity and host specificity in cycliophorans: a phylogeographic analysis along the North Atlantic and Mediterranean Sea. Molecular Ecology, 14, 4427–4440.
Obst, M., Funch, P., & Kristensen, R. M. (2006). A new species of Cycliophora from the mouthparts of the American lobster, Homarus americanus (Nephropidae, Decapoda). Organisms Diversity & Evolution, 6, 83–97.
Wanninger, A., & Neves, R. C. (2015) Cycliophora. In A. Wanninger (Ed.), Evolutionary developmental biology of invertebrates (Vol. 2: Lophotrochozoa (Spiralia), pp. 79–87). Wien: Springer-Verlag.
Acknowledgements
We are grateful to the generous support by the staff from The Sven Lovén Centre for Marine Sciences at the University of Gothenburg. The authors also thank Birgitte Rubak and Stine Elle (both Copenhagen) for providing the line drawings. We thank Kevin Kocot (University of Alabama, USA) for his helpful comments on a preliminary version of this article.
Author’s contributions
RCN and HR conceived of the study and participated in its design and coordination and drafted the manuscript. RCN is the main coordinator of the project, prepared the material for sequencing, coordinated the molecular genetic studies and analysed the data. SS analysed the data. JCG performed statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Supplementary Figure 2.
(A) Number of annotated contigs as a function of the stringency of the E-value threshold applied. Note that the lower the E-value, the more similar is the match between the contig and the annotated gene. (B) BLAST annotation of transcriptome assembly for E-values ≤1 × 10−5. Note that 14,541 contigs have high similarity with known proteins. (TIFF 166 kb)
Supplementary Figure 3.
Annotation of transcriptome assembly. Bar charts represent the level of similarity to proteins that belong to other (A) genera and (B) species. (TIFF 367 kb)
Supplementary Figure 4.
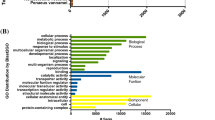
Annotation of transcriptome assembly. Bar charts represent the categorization of the contigs (E-values ≤1 × 10−5) into functional groups belonging to three main GO ontologies: (A) biological processes, (B) molecular functions and (C) cellular components. (TIFF 588 kb)
Supplementary Table 1.
List of all BLAST hits for the long open reading frames (ORF) predicted for the contigs present in the reference transcriptome. The table shows the following information for each of the long ORFs: Swiss-Prot accession and description of the protein hit, BLAST hit statistics such as percent identity, alignment length, mismatches, gap opens, E-value and bit score. The next columns for each ORF list associated IDs from different publicly available databases (EMBL, Ensembl and RefSeq). The last two columns of the table show the KEGG and GO IDs which could be associated with the Swissprot protein hit by the queried ORF. All IDs are formatted as hyperlinks into the respective databases for easy reference. (XLSX 4084 kb)
Supplementary Table 2.
List of all differentially expressed contigs/transcripts during the life cycle of Symbion pandora. We find 2660 genes that are significantly differentially expressed (P-adjusted <0.01) in the feeding stages from the asexual generation as compared to the sexual feeding stages. Among these, 1424 genes are upregulated in the sexual stages as compared to the asexual stages (upper part of the table). Conversely, 1236 genes are upregulated in the asexual stages as compared to the sexual stages (lower part of the table). Gene ontology (GO) class, description, and official GO identifier are listed for all the genes for which we were able to identify homologues in other species. Note that each contig appears as many times as it is assigned to a GO term. (XLSX 5031 kb)
Rights and permissions
About this article
Cite this article
Neves, R.C., Guimaraes, J.C., Strempel, S. et al. Transcriptome profiling of Symbion pandora (phylum Cycliophora): insights from a differential gene expression analysis. Org Divers Evol 17, 111–119 (2017). https://doi.org/10.1007/s13127-016-0315-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0315-1