Abstract
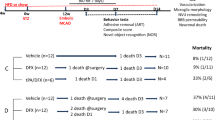
Diabetes worsens stroke outcome and increases the risk of hemorrhagic transformation (HT) after ischemic stroke, especially with tissue plasminogen activator (tPA) treatment. The widespread use of tPA is still limited by the fear of hemorrhagic transformation (HT), and underlying mechanisms are actively being pursued in preclinical studies. However, experimental models use a 10 times higher dose of tPA than the clinical dose (10 mg/kg) and mostly employ only male animals. In this translational study, we hypothesized that low-dose tPA will improve the functional recovery after the embolic stroke in both control and diabetic male and female animals. Diabetes was induced in age-matched male and female Wistar rats with high fat diet and low-dose streptozotocin (30 mg/kg, i.p.). Embolic stroke was induced with clot occlusion of the middle cerebral artery (MCA). The animals were treated with or without tPA (1 mg/kg, i.v.) at 90 min after surgery. An additional set of animals were subjected to 90 min MCAO with suture. Neurological deficits (composite score and adhesive removal test-ART), infarct size, edema ratio, and HT index were assessed 3 days after surgery. In the control groups, female rats had smaller infarcts and better functional outcomes. tPA decreased infarct size in both sexes with a greater effect in males. While there was no difference in HT between males and females without tPA, HT was less in the female + tPA group. In the diabetic groups, neuronal injury increased in females reaching that of the infarct sizes seen in male rats. tPA decreased infarct size in females but not males. HT was greater in female rats than in males and was not further increased with tPA. Diabetes worsened neurological deficits in both sexes. Male animals showed improved sensorimotor skills, especially with tPA treatment, but there was no improvement in females. These data suggest that diabetes amplifies neurovascular injury and neurological deficits in both sexes. Human dose tPA offers some degree of protection in male but not female rats. Given that control female animals experience less injury compared to male rats, the diabetes effect is more profound in females.







Similar content being viewed by others
References
Baird TA, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34(9):2208–14.
Ergul A, et al. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets. 2012;12(2):148–58.
Yan T, et al. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46(9):2599–606.
Ning R, et al. Tissue plasminogen activator treatment of stroke in type-1 diabetes rats. Neuroscience. 2012;222:326–32.
Ye X, et al. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–45.
Li W, et al. Comparative analysis of the neurovascular injury and functional outcomes in experimental stroke models in diabetic Goto-Kakizaki rats. Brain Res. 2013;1541:106–14.
Hafez S, et al. Comparative analysis of different methods of ischemia/reperfusion in hyperglycemic stroke outcomes: interaction with tPA. Transl Stroke Res. 2015;6(3):171–80.
Fan X, et al. Early insulin glycemic control combined with tPA thrombolysis reduces acute brain tissue damages in a focal embolic stroke model of diabetic rats. Stroke. 2013;44(1):255–9.
Fan X, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43(2):567–70.
Won SJ, et al. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Ann Neurol. 2011;70(4):583–90.
Haelewyn B, Risso JJ, Abraini JH. Human recombinant tissue- plasminogen activator (alteplase): why not use the ‘human’ dose for stroke studies in rats? J Cereb Blood Flow Metab. 2010;30(5):900–3.
Manwani B, et al. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35(2):221–9.
Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2013;63(2):238–53.
Bejot Y, Giroud M. Stroke in diabetic patients. Diabetes Metab. 2010;36(Suppl 3):S84–7.
Zhang L, et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113(1):85–95.
Vannucci SJ, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21(1):52–60.
Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4(3):279–85.
Landis SC, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–91.
Zhang RL, et al. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766(1–2):83–92.
Hoda MN, et al. Sex-independent neuroprotection with minocycline after experimental thromboembolic stroke. Exp Transl Stroke Med. 2011;3(1):16.
Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97.
Ergul A, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33.
Qin Z, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38(4):1362–7.
Kelly-Cobbs AI, et al. Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304(6):H806–15.
Prakash R, et al. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke. 2013;44(10):2875–82.
Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217(4562):855–7.
Kelly-Cobbs AI, et al. Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304(6):806–15.
Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39(5):711–6.
Fisher M, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–50.
Yan T, et al. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol Dis. 2012;46(1):157–64.
Li Q, et al. Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size. Stroke. 2013;44(11):3183–8.
Chen J, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42(12):3551–8.
Elgebaly MM, et al. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30(12):1928–38.
Ding G, et al. Persistent cerebrovascular damage after stroke in type two diabetic rats measured by magnetic resonance imaging. Stroke. 2015;46(2):507–12.
Reed MJ, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49(11):1390–4.
Ding SY, et al. Pioglitazone can ameliorate insulin resistance in low-dose streptozotocin and high sucrose-fat diet induced obese rats. Acta Pharmacol Sin. 2005;26(5):575–80.
Zhang M, et al. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045.
Wang C, et al. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620(1–3):131–7.
Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46(2):561–5.
Bushnell CD, et al. Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke. 2006;37(9):2387–99.
Howe MD, McCullough LD. Prevention and management of stroke in women. Expert Rev Cardiovasc Ther. 2015;13(4):403–15.
Manwani B, et al. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–700.
Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Women's Health (Lond Engl). 2011;7(3):319–39.
Liu M, et al. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009;27(3):163–79.
Ahnstedt H, McCullough LD, Cipolla MJ. The importance of considering sex differences in translational stroke research. Transl Stroke Res. 2016;7(4):261–73.
Writing Group Members, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360.
Campbell SJ, et al. Contact factor mediated fibrinolysis is increased by the combined oral contraceptive pill. Br J Obstet Gynaecol. 1993;100(1):79–84.
Acknowledgements
Adviye Ergul is a Research Career Scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. This work was supported in part by the VA Merit Award (BX000347), VA Research Career Scientists Award, and NIH (R01NS083559) to Adviye Ergul, and VA Merit Award (BX000891) and NIH award (NS063965) to Susan C. Fagan. The contents do not represent the views of the Department of Veterans Affairs or the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed. All protocols were approved by the institutional animal care and use committee. This study was conducted in accordance with the National Institute of Health guidelines for the care and use of animals in research, and adhered to the current RIGOR guidelines for the translational research with respect to (1) blinding of the study, (2) randomization of treatment (intervention) groups, (3) power analysis, and (4) statistical analysis.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, W., Ward, R., Valenzuela, J.P. et al. Diabetes Worsens Functional Outcomes in Young Female Rats: Comparison of Stroke Models, Tissue Plasminogen Activator Effects, and Sexes. Transl. Stroke Res. 8, 429–439 (2017). https://doi.org/10.1007/s12975-017-0525-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0525-7