Abstract
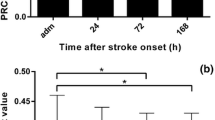
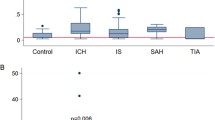
Dipeptidyl peptidase IV (DPPIV) inhibition may be a promising therapeutic strategy for acute stroke treatment, given its potential to prolong the biological half-life of neuroprotective substrates. A related protease, fibroblast activation protein (FAP), was recently shown to inactivate the same substrates. Therefore, it should also be investigated as a potential target in stroke. The study aimed to investigate whether stroke severity and outcome correlate with DPPIV and FAP activities and their kinetics shortly after acute ischemic stroke. DPPIV and FAP activities were analyzed in the serum of 50 hyperacute stroke patients at admission, 1 day, 3 days, and 7 days after stroke onset and in 50 age-matched healthy controls. This was done as part of the Middelheim’s Interdisciplinary Stroke Study. DPPIV activity tended to increase shortly after stroke compared to the control population. DPPIV and FAP activities steadily decreased in the first week after stroke onset. Higher infarct volumes (≥5 ml) and a more severe stroke (NIHSS >7) at admission were correlated with a stronger decrease in the activities of both enzymes. Moreover, these patients more often developed a progressive stroke, were more often institutionalized. Patients with a stronger increase in DPPIV activity at admission and decrease in the activity of both DPPIV and FAP during the first week after stroke onset had a more severe stroke and worse short-term outcomes.




Similar content being viewed by others
References
Darsalia V, Ortsäter H, Olverling A, Darlöf E, Wolbert P, Nyström T, Klein T, Sjöholm Å, Patrone C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes. 2013;62:1289–96.
Monami M, Ahrén B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:112–20.
Shannon RP. DPP-4 inhibition and neuroprotection: do mechanisms matter? Diabetes. 2013;62:1029–31.
Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–34.
Paczkowska E, Kucia M, Koziarska D, et al. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–44.
Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, Ellinor P, Furie KL. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43:441–5.
Keane FM, Nadvi NA, Yao T-W, Gorrell MD. Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-α. FEBS J. 2011;278:1316–32.
Röhnert P, Schmidt W, Emmerlich P, et al. Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN-like proteases in cerebral ischemia. J Neuroinflammation. 2012;9:44.
Matheeussen V, Jungraithmayr W, De Meester I. Dipeptidyl peptidase 4 as a therapeutic target in ischemia/reperfusion injury. Pharmacol Ther. 2012;136:267–82.
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41.
Brouns R, Heylen E, Willemse JL, Sheorajpanday R, De Surgeloose D, Verkerk R, De Deyn PP, Hendriks DF. The decrease in procarboxypeptidase U (TAFI) concentration in acute ischemic stroke correlates with stroke severity, evolution and outcome. J Thromb Haemost. 2010;8:75–80.
Brouns R, Sheorajpanday R, Kunnen J, De Surgeloose D, De Deyn PP. Clinical, biochemical and neuroimaging parameters after thrombolytic therapy predict long-term stroke outcome. Eur Neurol. 2009;62:9–15.
Brouns R, Sheorajpanday R, Wauters A, De Surgeloose D, Mariën P, De Deyn PP. Evaluation of lactate as a marker of metabolic stress and cause of secondary damage in acute ischemic stroke or TIA. Clin Chim Acta. 2008;397:27–31.
Brouns R, Marescau B, Possemiers I, Sheorajpanday R, De Deyn PP. Dimethylarginine levels in cerebrospinal fluid of hyperacute ischemic stroke patients are associated with stroke severity. Neurochem Res. 2009;34:1642–9.
Kehoe K, Brouns R, Verkerk R, Engelborghs S, De Deyn PP, Hendriks D, De Meester I. Prolyl carboxypeptidase activity decline correlates with severity and short-term outcome in acute ischemic stroke. Neurochem Res. 2015;40:81–8.
Birschel P, Ellul J, Barer D. Progressing stroke: towards an internationally agreed definition. Cerebrovasc Dis. 2004;17:242–52.
Matheeussen V, Lambeir A-M, Jungraithmayr W, Gomez N, Mc Entee K, Van der Veken P, Scharpé S, De Meester I. Method comparison of dipeptidyl peptidase IV activity assays and their application in biological samples containing reversible inhibitors. Clin Chim Acta. 2012;413:456–62.
Yang D, Nakajo Y, Iihara K, Kataoka H, Yanamoto H. Alogliptin, a dipeptidylpeptidase-4 inhibitor, for patients with diabetes mellitus type 2, induces tolerance to focal cerebral ischemia in non-diabetic, normal mice. Brain Res. 2013;1517:104–13.
Röhrborn D, Eckel J, Sell H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 2014;588:3870–7.
Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–25.
Borlongan CV, Glover LE, Sanberg PR, Hess DC. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Curr Pharm Des. 2012;18:3670–6.
Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Ther. 2003;306:948–53.
Kastin AJ, Akerstrom V. Nonsaturable entry of neuropeptide Y into brain. Am J Phys. 1999;276:E479–82.
Casrouge A, Decalf J, Ahloulay M, et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest. 2011;121:308–17.
Olsen TS. Blood glucose in acute stroke. Expert Rev Neurother. 2009;9:409–19.
Jackson EK, Mi Z, Tofovic SP, Gillespie DG. Effect of dipeptidyl peptidase 4 inhibition on arterial blood pressure is context dependent. Hypertension. 2015;65:238–49.
Jacob M, Chang L, Puré E. Fibroblast activation protein in remodeling tissues. Curr Mol Med. 2012;12:1220–43.
Brokopp CE, Schoenauer R, Richards P, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J. 2011;32:2713–22.
Acknowledgments
We would like to thank Jill Luyckx (Institute Born-Bunge, Antwerp) for her logistic support with the serum samples and Nicole Lamoen (University of Antwerp) for her technical assistance. In addition, we are grateful to the Institute Born-Bunge and its Antwerp Biobank for providing the samples from the Middelheim Interdisciplinary Stroke Study project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was supported by the University of Antwerp Research Fund (Grant FFB3551), the Institute Born-Bunge, the Antwerp Biobank, the Interuniversity Attraction Poles (IAP) program of the Belgian Science Policy Office, and the Flemish Government Methusalem excellence program, Belgium.
Conflict of Interest
The authors have no conflict of interest to declare. This study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committees of ZNA Antwerp and the University of Antwerp. For the stroke patients of ZNA Middelheim hospital, written informed consent was obtained from each participating subject or proxy in case of reduced consciousness. The (leftover) control samples from the UZA hospital were number coded and were obtained according to the FDA “Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens that are Not Individually Identifiable.”
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Baerts, L., Brouns, R., Kehoe, K. et al. Acute Ischemic Stroke Severity, Progression, and Outcome Relate to Changes in Dipeptidyl Peptidase IV and Fibroblast Activation Protein Activity. Transl. Stroke Res. 8, 157–164 (2017). https://doi.org/10.1007/s12975-016-0493-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0493-3