Abstract
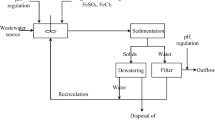
The electrochemical oxidation of an actual waste which comes from a chemical plant was studied. This effluent is generated in a polymerization unit and is mainly composed of a great variety of organics (including a complex mixture of water-soluble polymers of very different molecular weights) and solvents. The high variability of the organic load in industrial waste stream (4,000–20,000 mg dm−3 of chemical oxygen demand (COD)) makes the study of the system difficult. The results show that the key for an efficient electrolytic treatment is the selection of the anode material. The replacement of dimensional stable anodes by non-active electrodes is technically feasible as it leads to a very efficient process in terms of COD and total organic carbon removal. However, the use of PbO2 favours the release of toxic lead ions to the reaction medium. The efficiencies seem to depend on the pH and supporting electrolyte. This does not seem to be related to the electrochemical process, but to the oxidizability of the pollutant (mainly polymers) that should strongly depend on the pH. It is suspected that some functional groups of the polymer were more easily attacked by reagents electrogenerated under extreme pH values and that hypochlorite is less effective than persulphate. Regarding economic viability, electrochemical oxidation with a boron-doped diamond electrode can be used in an economically adequate way for the pretreatment of waste, but the energy cost necessary to deal with mass transfer limitations made this technique unsuitable for its use in a refining process of the quality of an effluent.







Similar content being viewed by others
References
K. Rajeshwar, J.G. Ibanez, G.M. Swain, Electrochemistry and environment. J. Appl. Electrochem. 24, 1077 (1994)
G. Chen, Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 38, 11 (2004)
C.A. Martínez-Huitle, E. Brillas, Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl. Catal. B: Environ. 87, 105 (2009)
G. Chen, X. Chen, P.L. Yue, Electrochemical behavior of novel Ti/IrO x –Sb2O5–SnO2 anodes. J. Phys. Chem. B 106, 4364 (2002)
C.A. Martinez-Huitle, M.A. Quiroz, C. Comninellis, S. Ferro, A. De Battisti, Electrochemical incineration of chloranilic acid using Ti/IrO2, Pb/PbO2 and Si/BDD electrodes. Electrochim. Acta 50, 949 (2004)
C.A. Martinez-Huitle, S. Ferro, A. De Battisti, Electrochemical incineration of oxalic acid: role of electrode material. Electrochim. Acta 49, 4027 (2004)
I. Sirés, E. Brillas, G. Cerisola, M. Panizza, Comparative depollution of mecoprop aqueous solutions by electrochemical incineration using BDD and PbO2 as high oxidation power anodes. J. Electroanal. Chem. 613, 151 (2008)
L. Ciríaco, C. Anjo, J. Correia, M.J. Pacheco, A. Lopes, Electrochemical degradation of ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim. Acta 54, 1464 (2009)
Y. Kong, Z.-L. Wang, Y. Wang, J. Yuan, Z.-D. Chen, Degradation of methyl orange in artificial wastewater through electrochemical oxidation using exfoliated graphite electrode. New Carbon Mat. 26(459) (2011)
B. Xue, Y. Zhang, J.Y. Wang, Electrochemical oxidation of bisphenol A on Ti/SnO2–Sb2O5/PbO2 anode for waste water treatment. Procedia Env. Sci. 10, 647 (2011)
Y. Zheng, W. Su, S. Chen, X. Wu, X. Chen, Ti/SnO2–Sb2O5–RuO2/α-PbO2/β-PbO2 electrodes for pollutants degradation. Chem. Eng. J. 174, 304 (2011)
E. Turro, A. Giannis, R. Cossu, E. Gidarakos, D. Mantzavinos, A. Katsaounis, Electrochemical oxidation of stabilized landfill leachate on DSA electrodes. J. Hazard. Mater. 190, 460 (2011)
P. Cañizares, J. Lobato, R. Paz, M.A. Rodrigo, C. Sáez, Electrochemical oxidation of phenolic compound wastes with BDD anodes. Water Res. 39, 2687 (2005)
I. Sirés, P.L. Cabot, F. Centellas, J.A. Garrido, R.M. Rodríguez, C. Arias, Electrochemical degradation of clofibric acid in water byanodic oxidation. Comparative study with platinum and boron doped diamond electrodes. Electrochim. Acta 52, 75 (2006)
P. Cañizares, R. Paz, J. Lobato, C. Sáez, M.A. Rodrigo, Electrochemical treatment of the effluent of a fine chemical manufacturing plant. J. Hazard. Mater. B138, 173 (2006)
P. Cañizares, R. Paz, C. Sáez, M.A. Rodrigo, Electrochemical oxidation of alcohols and carboxylic acids with diamond anodes: a comparison with other advanced oxidation processes. Electrochim. Acta 53, 2144 (2008)
M. Panizza, G. Cerisola, Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 109, 6541 (2009)
M. Panizza, G. Cerisola, Electrochemical degradation of gallic acid on a BDD anode. Chemosphere 77, 1060 (2009)
C. Flox, C. Arias, E. Brillas, A. Savall, K. Groenen-Serrano, Electrochemical incineration of cresols: a comparative study between PbO2 and boron-doped diamond anodes. Chemosphere 74, 1340 (2009)
M. Mascia, A. Vacca, A.M. Polcaro, S. Palmas, J. Rodriguez-Ruiz, A. Da Pozzo, Electrochemical treatment of phenolic waters in presence of chloride with boron-doped diamond (BDD) anodes: experimental study and mathematical model. J. Hazard. Mater. 174, 314 (2010)
N. Oturan, M. Hamza, S. Ammar, R. Abdelhédi, M.A. Oturan, Oxidation/mineralization of 2-nitrophenol in aqueous medium by electrochemical advanced oxidation processes using Pt/carbon-felt and BDD/carbon-felt cells. J. Electroanal. Chem. 661, 66 (2011)
E. Chatzisymeon, A. Dimou, D. Mantzavinos, A. Katsaounis, Electrochemical oxidation of model compounds and olive mill wastewater over DSA electrodes: 1. The case of Ti/IrO2 anode. J. Hazard. Mater. 167, 268 (2009)
E. Lacasa, J. Llanos, P. Cañizares, M.A. Rodrigo, Electrochemical denitrification with chlorides using DSA and BDD anodes. Chem. Eng. J. 184, 66 (2012)
APHA-AWWA-WPCF. Standard methods for the examination of water and wastewater (20th ed), edited by Clesceri, L.S., Greenberg, A.E., Eaton, A.D. and Franson, M.A.H. Washington DC: American Public Health Association, 1998.
M.A. Rodrigo, P. Cañizares, A. Sánchez-Carretero, C. Sáez, Use of conductive-diamond electrochemical oxidation for wastewater treatment. Catal. Today 151(1–2), 173 (2010)
B. Marselli, J. García-Gómez, P.A. Michaud, M.A. Rodrigo, C. Comninellis, Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J. Electrochem. Soc. 150(3), 79 (2003)
C. Comninellis, Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 39, 1857 (1994)
G. Foti, D. Gandini, C. Comninellis, A. Perret, W. Haenni, Oxidation of organics by intermediates of water discharge on IrO2 and synthetic diamond anodes. Electrochem. Solid-State Lett. 2, 228 (1999)
K. Serrano, P.A. Michaud, C. Comninellis, A. Savall, Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim. Acta 48, 431 (2002)
P. Cañizares, F. Larrondo, J. Lobato, M.A. Rodrigo, C. Sáez, Electrochemical synthesis of peroxodiphosphate using boron-doped diamond anodes. J. Electrochem. Soc. 152(11), D191 (2005)
A.M. Polcaro, A. Vacca, M. Mascia, F. Ferrara, Product and by-product formation in electrolysis of dilute chloride solutions. J. Appl. Electrochem. 38, 979 (2008)
P. Cañizares, C. Sáez, A. Sánchez-Carretero, M.A. Rodrigo, Synthesis of novel oxidants by electrochemical technology. J. Appl. Electrochem. 39(11), 2143 (2009)
P. Cañizares, R. Paz, C. Sáez, M.A. Rodrigo, Costs of the electrochemical oxidation of wastewaters: a comparison with ozonation and Fenton oxidation processes. J. Env. Manag. 90(1), 410 (2009)
Author information
Authors and Affiliations
Corresponding author
Additional information
Cristina Sáez: ISE member
Rights and permissions
About this article
Cite this article
Sáez, C., Cañizares, P., Llanos, J. et al. The Treatment of Actual Industrial Wastewaters Using Electrochemical Techniques. Electrocatalysis 4, 252–258 (2013). https://doi.org/10.1007/s12678-013-0136-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-013-0136-3