Abstract
For a study of interactions between the cancer-associated fibroblasts (CAFs) and the putative prostate cancer stem cells (CSCs), we used a conditional Pten deletion mouse model of prostatic adenocarcinoma to isolate both CAF cultures and CSC-enriched cell fractions from the primary tumors. The CSC subpopulation exhibited a collective phenotype of Lin−/SCA-1hi/CD49fhi/p63 hi/CK5 hi/AR lo/CK18 lo/Survivin hi/Runx2 hi and contained cells with the ability to both self-renew and differentiate into basal and luminal cells in vitro. The spheroids generated from the CSC-enriched subpopulation mimicked the glandular structures that could be produced from a similarly isolated cell fraction from the normal mouse prostate. The efficiency of spheroid formation was found to be influenced differentially by the nature of the fibroblasts that were co-cultured in the 3-D system. The growth and differentiation properties of the CSCs were significantly more enhanced by factors released from CAFs relative to normal prostate fibroblasts (NPFs). Additionally, increased commitment to differentiation to the luminal cell lineage was noted when CAFs were present. When CSCs admixed with either CAFs or NPFs were examined for formation of prostatic glandular structures in renal grafts in vivo, the lesions formed were generally more in numbers in the presence of CAFs than NPFs. Furthermore, lesions formed with CAFs often displayed tumor-like complex histopathology and contained increased numbers of proliferating cells. Taken together, the results suggested that the CAFs in the prostate tumor microenvironment can contribute to the biologic properties of the CSCs and by this account may play a major role in prostate tumorigenesis and progression. Thus, it would be important now to identify the paracrine and/or juxtacrine factors that are responsible for the stimulation of the cancer stem cells.
Similar content being viewed by others
Introduction
It has been proposed that cancer contains a minor population of cells that can self-renew while simultaneously giving rise to tumor cells [1, 2]. Such cells may also mediate tumor homeostasis, progression, metastasis, and recurrence. These cells have been referred to as tumorigenic cells, tumor-initiating cells, or cancer stem cells (CSCs). Compared to CSCs, other tumor cells posses only limited proliferation and tumorigenic potentials. CSCs, first described in tumors of the hematopoietic system, were found to share certain cell surface marker phenotype with the normal hematopoietic stem cells [2]. In recent years, presence of a similar relationship between tissue stem cells and CSC fractions of solid tumors from various organs including the prostate is also being increasingly implicated [3–14].
All carcinoma tissues, in general, consist of malignant epithelial cells and their progenitors, a variety of stromal cells including fibroblasts (and myofibroblasts), endothelial cells, pericytes, and inflammatory cells. Extracellular proteins secreted by these different cell types are present in the tumor microenvironment. These constitute a complex array of growth factors, cytokines, chemokines, and adhesive molecules that are likely to alter the balance between proliferation, survival, differentiation, and quiescence in both cancer cell populations and their progenitors during tumor progression, metastasis, or recurrence. The important contribution of stroma to genesis and progression of a variety of tumors has been described [15–19]. Myofibroblasts, characterized by their expression of α-smooth muscle actin (α-SMA), which represent a large fraction of stromal fibroblastic cell populations in cancers, particularly in prostate and breast cancers, are also present in areas of wound healing and chronic inflammation. The myofibroblasts are known to stimulate epithelial cell growth through their ability to produce extracellular matrix and by secretion of growth factors and cytokines and to support angiogenesis [16, 17, 20].
There is evidence that myofibroblastic cells from prostate tumors, termed as “cancer-associated” fibroblasts (CAFs) can enhance the tumorigenic potential of the epithelial compartment [21, 22]. In this regard, an important new question is if fibroblasts acquire genetic and/or epigenetic alterations during tumor progression. Recently in a mouse model of prostate cancer, it has been demonstrated that a selective evolution of stromal mesenchyme does occur with p53 loss in response to epithelial tumorigenesis [23]. Such selection of aberrant stromal cells in some human epithelial cancers was earlier implicated in the cancer progression [24–28], although lack of detection of changes was also reported [29]. Inflammation and innate immunity that occur in the microenvironment are also widely recognized to play important roles in epithelial cancer progression [19, 30].
The mouse prostate has been the primary focus of research in regards to normal stem cells in the animal prostate. There are three epithelial cell types from which the prostate is composed: luminal cells, the differentiated secretory cells which are the major cell type; neuroendocrine cells, rare and morphologically heterogeneous cells present in both the basal and luminal cell layers; and basal cells, the undifferentiated, non-secretory cells that may contain the progenitor cells of the prostate [31, 32]. The proximal region attached to the urethra is the probable location of stem cells because it contains a high number of label-retaining cells, which express stem cell-specific markers, are resistant to androgen ablation, and have a greater capacity to regenerate prostate tissue in growth assays as compared to cells located in the distal regions [33, 34]. Stem cells reside in a specialized microenvironment, in which their quiescence and proliferation appear to be regulated by factors such as transforming growth factor-β, epidermal growth factor, insulin-like growth factor, etc. [32, 35, 36]. There is increasing evidence that cell markers such as stem cell antigen-1 (SCA-1), laminin receptor α6 integrin (CD49f), CD133 (prominin), CD44, and CD117 (c-kit, stem cell factor receptor) can be used to enrich for stem cells of the mouse prostate [32, 37, 38]. Interestingly, there is evidence in a mouse xenograft model that the α6 integrin, whose expression is upregulated in prostate cancer cells [39], can be converted to a novel form by cleavage of the laminin-binding domain from the cell surface by urokinase-type plasminogen activator, and such cleavage may permit extravasation of human prostate cancer cells from the circulation to bone [40, 41]. Another exciting new evidence is that a single mouse prostate stem cell defined by a SCA-1+ CD133+ CD44+ CD117+ phenotype and implanted under the renal capsule can generate secretion-producing prostatic ducts consisting of basal, luminal, and neuroendocrine cells [38].
In regard to modeling human prostate cancer in mice, transgenic mice with activation or deficiency of genes such as androgen receptor, fibroblast growth factor 8, c-Myc, Akt, Pten, Nkx3.1, Rb, p53, Apc, or some of their combinations demonstrate gene-dependent development of the various stages of prostate cancer [42–54]. Preliminary findings suggest that different genetic events have unequal transforming effects on stem cells and their progeny [55]. One mouse model [45] that closely recapitulates the human prostate cancer is the conditional Pten deletion mouse line (cPten −/−), which concerns prostate epithelium-specific loss of PTEN, a negative regulator of the phosphatidylinositol 3-kinase/AKT pathway. In humans, approximately 70% of primary prostate cancers exhibit a loss of at least one Pten allele, and functional suppression of both alleles is associated with advanced disease [56, 57]. In mice, Pten deletion of one allele leads to the development of high-grade prostate intracellular neoplasia [58], but loss of both alleles results in adenocarcinoma at age beginning from 9 weeks and invasive prostate cancer that metastasizes to primarily to lymph nodes subsequently [45]. The mouse models present the potential to increase our understanding of the relevance of various cellular components and signaling proteins that occur in the tumor microenvironment to the biology of prostate cancer stem cells in the progression and recurrence of the disease.
Results
Relevant Characteristics of the Tumor Model and Tumor-Derived Fibroblast Cultures
The utility of the conditional Pten deletion model [45], which we abbreviate as cPten −/−, was further improved by combining it with a conditional luciferase reporter line [54]. In this mouse model, the recombination mechanism that inactivates Pten alleles also activates the luciferase (L) reporter gene in the same cells. In this cPten −/− L model, the growth of the primary cancer can thus be followed noninvasively by bioluminescence imaging (BLI; Fig. 1). It is found that surgical castration of tumor-bearing animals leads to a reduced bioluminescence signal corresponding to tumor regression, and most notably, when castrated animals are maintained, the emergence of androgen depletion-independent (ADI) cancer [59] is detected using BLI. Detectable recurrence occurs at time periods, varying from 7 to 28 weeks post-castration. It is noteworthy that parameters of progression are similar to those of the human cancer. For example, one of the common features of recurrent prostate cancer is neuroendocrine differentiation. Human prostate adenocarcinomas exhibit at least focal positivity for neuroendocrine markers ranging from 30% to 100% of cases. Neuroendocrine differentiation has been reported to increase in advanced tumors and ADI tumors [60]. Our comparison of phenotypically distinct populations of epithelial cells in cancer tissues of the cPten −/− L mice indicates that in addition to well-established hyperplasia of CK5-positive basal cell compartment [61], Pten inactivation leads to the expansion of cells with neuroendocrine differentiation positive for synaptophysin A marker [54].
Genetic engineering of the cPten −/− L mouse strain. The schematic diagram shows that the cPten −/− mouse model was derived from combination of the PB-Cre4 [75] and Pten loxP [76] lines. PB-Cre4 line contains the Cre gene whose expression is driven by the ARR2PB [77] prostate epithelium-specific and androgen responsive promoter, and the Pten loxp line has two loxP sites flanking Pten Exon5, the phosphatase functional domain. This model [45] was further combined with another line with floxed luciferase allele, Cre–loxP recombination in which activates the reporter gene [78]. The resultant cPten −/− L strain [54] allows us to monitor tumor progression noninvasively through bioluminescence imaging
With the help of BLI technology, it is now possible to collect tumors from cPten −/− L mice at specific stages of prostate tumor progression: primary growth, regression, and recurrence (Fig. 2a) for the purpose obtaining specific cell types including epithelial cells and CAFs. For example, we recently reported the establishment and characterization of four novel mouse prostate cancer cell lines from primary (AD, androgen dependent) and recurrent (ADI, androgen depletion independent) prostate tumor [62] and described two primary cultures of CAFs, CAF-1, and CAF-2 from the AD tumor phase [63]. These CAFs present a myofibroblastic phenotype as they express α-SMA. Similarly, primary cultures have been derived from other AD and ADI tumors as well as normal mouse prostates. Cultures from the normal prostate are designated as normal prostate fibroblasts (NPFs). The expression of α-SMA is also detected in primary cultures of NPFs, although the levels are higher in CAFs relative to NPFs (Fig. 2b).
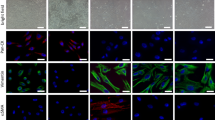
Isolation of CAFs from cPten −/− L tumors. a Schematic representation of monitoring of the cancer progression via bioluminescence imaging; tumor stages are indicated by 1 androgen-dependent growth phase, 2 regression phase, 3 regressed or dormant state, and 4 androgen depletion-independent growth phase. b Illustration of myofibroblastic phenotype of the stromal fibroblasts cultured from the tumors of the model or the normal counterpart tissues. Cells were fixed and examined for the expression of α-SMA by immunofluorescence. Practically, all cells appeared to be stained
Properties of the Tumor-Derived Cancer Stem Cell Subpopulation
SCA-1 is a marker that has been demonstrated to enrich for stem cells in normal mouse hematopoietic, skin, cardiac, and testicular tissues [64–67] and prostate epithelium [68]. In normal murine prostate, androgen deprivation leads to enrichment of SCA-1+ cell subpopulation in the proximal region, which is considered to be a niche for the resident stem cells [69]. There is evidence that Pten deletion may cause expansion of SCA-1+ cell subpopulation in the prostate tumors [61]. Recognizing that SCA-1+ cell fraction isolated from normal mouse prostate can self-renew and differentiate in vitro [69] and can regenerate prostate glandular structures in vivo [70], we applied a similar approach in attempts to isolate putative cancer stem cell subpopulation that might be present in the adenocarcinoma of cPten −/− L mice. A recent study also describes the utility of the SCA-1 marker for the enrichment of tumor stem/progenitor cells from the cPten −/− tumors of the prostate [71].
The strategy was to isolate tumor cell fractions free of non-prostatic cells (Ter119−, CD45−, and CD31−, collectively called Lin−) and enriched for SCA-1 marker, using fluorescence-activated cell sorting (FACS). Co-culture of such cells from the tumors (T-LS cells) in matrigel 3-D culture with either NPFs or CAFs that are grown in an insert leads to the formation of spheroids or prostaspheres [72]. The T-LS spheroids are composed of multiple basal and luminal cell layers, as detected by the positive co-immunofluorescence staining with CK8 (luminal cell marker) and p63 (basal cell marker). The T-LS spheroids produced in the co-culture with CAFs are generally of larger size containing multiple layers of luminal cells as compared to those with NPFs or urogenital sinus mesenchyme (UGSM). It is also noted that in T-LS spheroids, the percentage of CK8+ luminal cells is higher when co-cultured with CAF than with NPF; however, the reverse is seen with the percentage of p63+ basal cells (Fig. 3). These results indicate that T-LS cell subpopulation may harbor CSCs, and CAFs may upregulate the different pathways affecting proliferation, self-renewal, and differentiation in CSCs.
Lineage-specific differentiation of the cancer progenitor cells is differentially influenced by the stromal fibroblasts. Percentages of p63+, CK8+, and double positive cells detected in spheroids formed in vitro from the co-culturing of the tumor progenitor LS cell fraction with either CAF or NPF fibroblasts. The number of positive cells was divided by the total number of DAPI-stained nuclei to determine the percentage. This figure was adapted and modified from [72], supplemental Fig. S1B
By using CD49f as an additional cell marker, CSC subpopulation can be further enriched. Lin− SCA-1+ CD49f+ (LSC) tumor cell subpopulation can be serially passaged in culture retaining the ability to form spheroids each time for at least up to five passages in the experimental conditions we use. LSC, not LSC−, populations demonstrate significant spheroid-forming ability [72]. Thus, cancer-derived LSC cells are determined to posses multiple stem/progenitor cell properties of self-renewal and differentiation in vitro. We then further enriched the subpopulation for CSC by collecting only a small group of cells that strongly express both SCA-1 and CD49f surface markers (LSChi), distinguishable from those with medium expression (LSCme). This isolation technique is illustrated in the diagram in Fig. 4a and FACS plot in Fig. 4b. The proportion of LSC cell subpopulation in the tumor is much higher than that in the normal tissues, and this trend also holds when LSChi cell subpopulation in tumor and normal tissues are compared [72].
Isolation of LSChi, LSCme, and LSC− subpopulations. a A schematic diagram shows the steps by which single cells isolated from mouse prostate tissues were first segregated into Lin+ and Lin− cell subpopulations, and then Lin− cells were separated into LSC and LSC− cells based on the expression levels of SCA-1 and CD49f. LSC cells with the highest CD49f and SCA-1 levels were labeled as LSChi, those with medium CD49f expression levels as LSCme, and others as LSC−. b Illustration of gating plots for FACS sorting of the single cells. Tumor cell fraction shown as T-Lin− cells (left) were further fractionated as T-LSChi, T-LSCme, and T-LSC− subfractions (right). c Adapted from Fig. 3c of our published work [72]. Analysis of the spheroid-forming ability of T-LSChi and T-LSCme cells in the 3D co-culture system with stromal cells of different sources. T-LSChi (104) or T-LSCme (104) cells, isolated from the same tumor tissue, were co-cultured with the same number of NPFs or CAFs for 14 days following which the number of spheroids formed were counted
It is noteworthy that T-LSChi cells are able to form spheroids while T-LSCme cells practically lack this property (Fig. 3c) [72]. Relative to T-LSCme, T-LSChi cells express higher RNA levels of basal cell markers (p63 and CK5) and lower RNA levels of luminal cell marker (CK18) and androgen receptor (AR; Fig. 5a). A similar observation is made from such cell subpopulations (N-LSChi, N-LSCme, and N-LSC−) collected from the normal mouse prostate (Fig. 5b). Relative to T-LSC− cells, higher expression levels of both Survivin and Runx2, but not Grp78, are detected in both T-LSCme and T-LSChi cells (Fig. 5a). LSChi cells or at least a majority of the cells present in this selected subpopulation also appear to harbor deletion of the Pten gene, suggesting that Cre-mediated recombination had occurred in these cells of the tumors (Fig. 5c). The lack of more complete gene deletion may be due to the presence of normal tissue stem cells as well other cellular contaminants in the LSChi fraction. The spheroid-forming efficiency of the LSChi cells is differentially influenced by the factors secreted from fibroblasts in co-cultures. As compared to NPFs, CAFs enhance spheroid formation by two-fold (Fig. 3c).
Gene expression analyses with LSC cell fractions. a RNA expression levels of CK5, p63, CK18, AR, CD44, CD133, Survivin, Runx2, and Grp78 in LSChi (T-hi), LSCme (T-me), and LSC− (T-none) were examined by real-time quantitative PCR. b Similar analyses of the corresponding cell fractions from normal prostate tissues. c Detection of Pten gene deletion in T-LSChi cells. Genomic DNA extracted from N-LSChi, T-LSC−, and T-LSChi cells were examined using primers specific to Pten exon5 DNA sequence by real-time PCR. Intact Pten allele level detected in N-LSChi cells was set to 100%. a and b were adapted and modified from Fig. 5 and c from Fig. 3d of our published work [72]
To examine the in vivo biological properties of the T-LSChi cells, we mixed these cells with UGSM, NPFs, or CAFs and transplanted them under the renal capsule of NOD.SCID male mice [72]. The incidence of glandular structure formation is 100% when T-LSChi cells are combined with UGSM or CAFs, but only 72% when with NPFs. Although no such structures were detected in the T-LSCme grafts, two of six grafts formed from T-LSC− cells contained small glandular structures (Table 1). While the grafts grown from the T-LSChi cells in the presence of fibroblasts are found to develop multiple glandular structures in most cases, grafts formed with CAFs, but not UGSM or NPFs, appear to have increased number of glandular structures (Fig. 6a), displaying larger areas covered by glandular structures (Fig. 6b) and increased tumor-like histopathologies [72]. Ki67 staining also shows that glandular structures in grafts containing LSChi cells and CAFs exhibit approximately three-folds more proliferating cells than those from NPF grafts (Fig. 7a, b). Immunostaining results show that epithelial cells expressing AR, CK8, and NKX3.1 are abundant within the structures along with a small number of CK5-positive cells. Based on these biological findings along with results of candidate gene expression analyses, we hypothesize that the CSC subpopulation may be identified by the following collective phenotype: Lin−/SCA-1hi/CD49fhi/p63hi/CK5hi/ARlo/CK18lo/Survivinhi/Runx2hi. Furthermore, these observations underscore a significant role of CAFs in CSC biology and open up possibilities for identifying the responsible factors and the mechanisms underlying the heterotypic cell–cell interactions implicated by our study.
Analyses of glandular structures formed in renal grafts. a Comparison of the number of glandular structures formed from T-LSChi cells with either NPFs or CAFs. Tissue sections were stained by H&E, and the numbers of detectable glandular structures in each graft were counted. Slides from grafts from the T-LSChi + NPFs and grafts from T-LSChi + CAFs, which were determined to harbor glandular structures, were evaluated for this purpose. b Comparison of the areas covered by the glandular structures in these same grafts. The areas were measured by using ImageJ software. These figures were adapted from [72], supplemental Fig. S2A, B
Comparison of the proliferation index in the glandular structures formed in the renal grafts. a Immunohistochemical staining for Ki67 in glandular structures formed from T-LSChi cells. The nuclear staining is indicated by black arrowheads. Positive cells were detected in glandular structures formed from co-inoculation with either NPFs (upper panel) or CAFs (lower panel). b Adapted from supplemental Fig. S2C from [72]. Ten glandular structures from each group, T-LSChi + CAFs and T-LSChi + NPFs, were analyzed for Ki67 staining and the percentage of positive cells calculated to compare the proliferative indices between NPFs and CAFs
Discussion
There is currently a high level of interest in tumor microenvironment with the emphasis placed on the host stroma in tumorigenesis and responses to treatment. To delineate mechanisms of tumor–stroma interactions in cancer, it may be necessary for practical considerations to focus on one stromal cellular compartment at a time. With the accumulation of information on each type of interactions, it may then be possible to deduce a more comprehensive understanding of the role of the stroma on the growth and progression of a specific cancer type. In this report, we describe a model system to investigate the influence of the myofibroblasts present in the prostate tumors on the potency of the putative CSCs from the same tumors. The emphasis is placed on CSCs, which constitute only a minor population because they may be central to homeostasis, progression, and recurrence of the tumors. For this initial work [72], myofibroblastic CAFs and the CSC were derived from the primary tumors of the conditional Pten deletion model of the mouse prostate adenocarcinoma.
We refined a procedure for the isolation of epithelial cell fractions retaining a small number of cells with properties of putative CSCs from the prostate tumor mouse model. A cell fraction from this tumor model is shown to possess self-renewal and spheroid-forming abilities along with multi-potentiality for differentiation in vitro and the ability to form tumor-like glandular structures in vivo under appropriate conditions. We have observed a significant difference in the pattern of relative expression of certain relevant genes in the subpopulation of epithelial cells with which the functional CSCs segregate. The expressions of basal cell markers CK5 and p63 are strong in CSCs, while those of CK18 and AR are low. The same general pattern is found in the respective subpopulation from the normal mouse prostate. A high level of expression is also noted for two cancer-related genes: Survivin and Runx2 in CSCs. This finding is especially interesting since we have documented a strong correlation between increased levels of Survivin and Runx2 with the growth of the tumor in the model [73]. Moreover, Runx2 appears to be a major regulator of Survivin gene transcription in prostate cancer cells [74].
We demonstrate that the in vitro spheroid-forming efficiency of the CSC-enriched cells is differentially influenced by the fibroblasts in co-cultures. The modified spheroid-forming co-culture system we used has the promise to be a powerful method to facilitate the studies of paracrine signaling in interactions between stromal fibroblasts and CSCs. As compared to UGSM or NPFs, CAFs enhance spheroid formation by two-fold. It also appears that kinetics of differentiation toward the luminal cell phenotype over basal cell type is favored in the presences of CAFs relative to the other fibroblastic cells. In vivo, the grafts grown from CSCs are found to contain multiple glandular structures in each case, although grafts formed with CAFs appear to exhibit higher proliferative index and increased number of lesions with more complex morphology as compared to those formed with NPFs. These observations with CAFs underscore a significant role of CAFs in CSC biology and motivate studies to uncover the responsible molecular interactions. It is likely that many different molecules with positive or opposite effects may act in concert to modulate the complex processes of CSC biology. Thus, analysis of each implicated factor alone and in specific combinations will be needed to dissect parameters that converge to drive the cascade to sustain or stimulate CSC properties. Identification of pertinent secreted proteins from the model system will then lead to the scrutiny of analogous factors on human prostate CSCs and their distribution in the stromal compartments in prostate cancer specimens from different stages of the disease.
It is expected that with a better understanding of the signaling between CSCs and CAFs, pathway-specific inhibitors that block CAF–CSC interactions could be designed and tested on the preclinical model systems to better inform clinical development. Ultimate goal of these studies would be to attack prostate cancer at its core, which is the persistence and increased virulence of CSCs with the disease progression. Moreover, a focus on stromal fibroblasts that regulate prostate CSC activity offers a new direction for therapeutic development against a cellular compartment different from the malignant epithelium.
Conclusion
Evidence is presented by using a prostatic adenocarcinoma mouse model to support the notion that, like stem cells of the normal mouse prostate gland, a minor population of epithelial cells exists in the prostate cancer tissue that harbors the potential for both self-renewal and multi-potential differentiation activity. This cellular subpopulation, tentatively designated as cancer stem cells, may indeed be a renewable source for cancer persistence, growth, and re-growth or recurrence. It is also described that both the cell biology and the abnormal behavior of these stem cell-like cells can be robustly influenced by the myofibroblasts that occur in the tumor microenvironment. The characterization of the factors involved in the implicated heterotypic cell–cell interactions may provide new insights to strategies to improve prostate cancer treatment by targeting not only the epithelial compartment but also a fibroblastic compartment of the tumor microenvironment.
References
Al-Hajj M, Clarke MF (2004) Self-renewal and solid tumor stem cells. Oncogene 23:7274–7282
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Isaacs JT, Coffey DS (1989) Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 2:33–50
Signoretti S, Pires MM, Lindauer M et al (2005) p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci USA 102:11355–11360
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Al-Hajj M, Wicha MS, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988
Ricci-Vitiani L, Lombardi DG, Pilozzi E et al (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115
Kim CF, Jackson EL, Woolfenden AE et al (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823–835
Ma S, Chan KW, Hu L et al (2007) Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 132:2542–2556
Li C, Heidt DG, Dalerba P et al (2007) Identification of pancreatic cancer stem cells. Cancer Res 67:1030–1037
Ferrandina G, Bonanno G, Pierelli L et al (2008) Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer 18:506–514
Prince ME, Sivanandan R, Kaczorowski A et al (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 104:973–978
Fang D, Nguyen TK, Leishear K et al (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65:9328–9337
Collins AT, Berry PA, Hyde C et al (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951
Mbeunkui F, Johann DJ Jr (2009) Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 63:571–582
Hu M, Polyak K (2008) Microenvironmental regulation of cancer development. Curr Opin Genet Dev 18:27–34
Mueller MM, Fusenig NE (2004) Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4:839–849
Orimo A, Weinberg RA (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5:1597–1601
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Olumi AF, Grossfeld GD, Hayward SW et al (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59:5002–5011
Cunha GR, Hayward SW, Wang YZ et al (2003) Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 107:1–10
Hill R, Song Y, Cardiff RD et al (2005) Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 123:1001–1011
Fukino K, Shen L, Matsumoto S et al (2004) Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res 64:7231–7236
Kurose K, Gilley K, Matsumoto S et al (2002) Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet 32:355–357
Matsumoto N, Yoshida T, Yamashita K et al (2003) Possible alternative carcinogenesis pathway featuring microsatellite instability in colorectal cancer stroma. Br J Cancer 89:707–712
Moinfar F, Man YG, Arnould L et al (2000) Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res 60:2562–2566
Tuhkanen H, Anttila M, Kosma VM et al (2004) Genetic alterations in the peritumoral stromal cells of malignant and borderline epithelial ovarian tumors as indicated by allelic imbalance on chromosome 3p. Int J Cancer 109:247–252
Allinen M, Beroukhim R, Cai L et al (2004) Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6:17–32
de Visser KE, Korets LV, Coussens LM (2005) De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7:411–423
Nikitin AY, Matoso A, Roy-Burman P (2007) Prostate stem cells and cancer. Histol Histopathol 22:1043–1049
Nikitin A, Nafus M, Zhou Z et al (2009) Prostate stem cells and cancer in animals. Stem cells and cancer. Humana, New York, pp 199–216
Tsujimura A, Koikawa Y, Salm S et al (2002) Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol 157:1257–1265
Goto K, Salm SN, Coetzee S et al (2006) Proximal prostatic stem cells are programmed to regenerate a proximal–distal ductal axis. Stem Cells 24:1859–1868
Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116:769–778
Salm SN, Burger PE, Coetzee S et al (2005) TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol 170:81–90
Lawson DA, Xin L, Lukacs R et al (2005) Prostate stem cells and prostate cancer. Cold Spring Harb Symp Quant Biol 70:187–196
Leong KG, Wang BE, Johnson L et al (2008) Generation of a prostate from a single adult stem cell. Nature 456:804–808
Goel HL, Li J, Kogan S et al (2008) Integrins in prostate cancer progression. Endocr Relat Cancer 15:657–664
Demetriou MC, Pennington ME, Nagle RB et al (2004) Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer. Exp Cell Res 294:550–558
Ports MO, Nagle RB, Pond GD et al (2009) Extracellular engagement of alpha6 integrin inhibited urokinase-type plasminogen activator-mediated cleavage and delayed human prostate bone metastasis. Cancer Res 69:5007–5014
Abate-Shen C, Shen MM (2002) Mouse models of prostate carcinogenesis. Trends Genet 18:S1–S5
Huang J, Powell WC, Khodavirdi AC et al (2002) Prostatic intraepithelial neoplasia in mice with conditional disruption of the retinoid X receptor alpha allele in the prostate epithelium. Cancer Res 62:4812–4819
Song Z, Wu X, Powell WC et al (2002) Fibroblast growth factor 8 isoform B overexpression in prostate epithelium: a new mouse model for prostatic intraepithelial neoplasia. Cancer Res 62:5096–5105
Wang S, Gao J, Lei Q et al (2003) Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4:209–221
Ellwood-Yen K, Graeber TG, Wongvipat J et al (2003) Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4:223–238
Abdulkadir SA, Magee JA, Peters TJ et al (2002) Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 22:1495–1503
Roy-Burman P, Wu H, Powell WC et al (2004) Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer 11:225–254
Chen Z, Trotman LC, Shaffer D et al (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730
Zhou Z, Flesken-Nikitin A, Corney DC et al (2006) Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res 66:7889–7898
Zhong C, Saribekyan G, Liao CP et al (2006) Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis. Cancer Res 66:2188–2194
Abate-Shen C (2006) A new generation of mouse models of cancer for translational research. Clin Cancer Res 12:5274–5276
Bruxvoort KJ, Charbonneau HM, Giambernardi TA et al (2007) Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res 67:2490–2496
Liao CP, Zhong C, Saribekyan G et al (2007) Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res 67:7525–7533
Zhou Z, Flesken-Nikitin A, Nikitin AY (2007) Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res 67:5683–5690
Shen MM, Abate-Shen C (2007) Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res 67:6535–6538
Carver BS, Pandolfi PP (2006) Mouse modeling in oncologic preclinical and translational research. Clin Cancer Res 12:5305–5311
Podsypanina K, Ellenson LH, Nemes A et al (1999) Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96:1563–1568
Roy-Burman P, Tindall DJ, Robins DM et al (2005) Androgens and prostate cancer: are the descriptors valid? Cancer Biol Ther 4:4–5
Abrahamsson PA (1999) Neuroendocrine differentiation in prostatic carcinoma. Prostate 39:135–148
Wang S, Garcia AJ, Wu M et al (2006) Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA 103:1480–1485
Liao CP, Liang M, Cohen MB et al (2010) Mouse prostate cancer cell lines established from primary and post-castration recurrent tumors. Horm Cancer 1:44–54
Yang S, Pham LK, Liao CP et al (2008) A novel bone morphogenetic protein signaling in heterotypic cell interactions in prostate cancer. Cancer Res 68:198–205
Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241:58–62
Montanaro F, Liadaki K, Volinski J et al (2003) Skeletal muscle engraftment potential of adult mouse skin side population cells. Proc Natl Acad Sci USA 100:9336–9341
Falciatori I, Borsellino G, Haliassos N et al (2004) Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. FASEB J 18:376–378
Matsuura K, Nagai T, Nishigaki N et al (2004) Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 279:11384–11391
Burger PE, Xiong X, Coetzee S et al (2005) Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA 102:7180–7185
Xin L, Lawson DA, Witte ON (2005) The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA 102:6942–6947
Lawson DA, Xin L, Lukacs RU et al (2007) Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 104:181–186
Mulholland DJ, Xin L, Morim A et al (2009) Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res 69:8555–8562
Liao CP, Adisetiyo H, Liang M et al (2010) Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res 70:7294–7303
Yang S, Lim M, Pham LK et al (2006) Bone morphogenetic protein 7 protects prostate cancer cells from stress-induced apoptosis via both Smad and c-Jun NH2-terminal kinase pathways. Cancer Res 66:4285–4290
Lim M, Zhong C, Yang S et al (2010) Runx2 regulates survivin expression in prostate cancer cells. Lab Invest 90:222–233
Wu X, Wu J, Huang J et al (2001) Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 101:61–69
Lesche R, Groszer M, Gao J et al (2002) Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32:148–149
Zhang J, Thomas TZ, Kasper S et al (2000) A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology 141:4698–4710
Lyons SK, Meuwissen R, Krimpenfort P et al (2003) The generation of a conditional reporter that enables bioluminescence imaging of Cre/loxP-dependent tumorigenesis in mice. Cancer Res 63:7042–7046
Acknowledgments
This work was supported by the National Institutes of Health research grants RO1 CA59705 and RO1 CA113392 to P. Roy-Burman and, in part, by supports to C-P. Liao and H. Adisetiyo from the California Institute for Regenerative Medicine training grant T1-004.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, CP., Adisetiyo, H., Liang, M. et al. Cancer Stem Cells and Microenvironment in Prostate Cancer Progression. HORM CANC 1, 297–305 (2010). https://doi.org/10.1007/s12672-010-0051-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-010-0051-5