Abstract
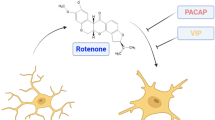
There has been a growing recognition of the role of neuroinflammation caused by microglia-exaggerated release of inflammatory mediators in the pathogenesis of Parkinson’s disease (PD). Pituitary adenylate cyclase activating polypeptide (PACAP) is an endogenous 38 amino acid containing neuropeptide that has been shown to possess neurotrophic as well as neuroprotective properties. In this study, we sought to determine whether PACAP could protect SH-SY5Y dopaminergic cells against toxicity induced by inflammatory mediators. For this purpose, THP-1 cells which possess microglia-like property were stimulated by a combination of lipopolysaccharide (LPS) and interferon gamma (IFN-γ), and the media containing inflammatory mediators were isolated and applied to SH-SY5Y cells. Such treatment resulted in approximately 54 % cell death as well as a reduction in brain-derived neurotrophic factor (BDNF) and phosphorylated cyclic AMP response element-binding protein (p-CREB). Pretreatment of the SH-SY5Y cells with PACAP (1-38) dose-dependently attenuated toxicity induced by the inflammatory mediators. PACAP effects, in turn, were dose-dependently blocked by the PACAP receptor antagonist (PACAP 6-38). These results suggest protective effects of PACAP against inflammatory-induced toxicity in a cellular model of PD that is likely mediated by enhancement of cell survival markers through activation of PACAP receptors. Hence, PACAP or its agonists could be of therapeutic benefit in inflammatory-mediated PD.







Similar content being viewed by others
References
Barcia C, Fernandez Barreiro A, Poza M, Herrero MT (2003) Parkinson’s disease and inflammatory changes. Neurotox Res 5:411–418
Beatie MS (2004) Inflammation and apoptosis: Linked therapeutic targets in spinal cord injury. Trends Mol Med 10:580–583
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012:845618
Botia B, Jolivel V, Burel D, Le Joncour V, Roy V, Naassila M, Bénard M, Fournier A, Vaudry H, Vaudry D (2011) Neuroprotective effects of PACAP against ethanol-induced toxicity in the developing rat cerebellum. Neurotox Res 19:423–434
Brown D, Tamas A, Reglodi D, Tizabi Y (2013) PACAP protects against salsolinol-induced toxicity in dopaminergic SH-SY5Y cells: implication for Parkinson’s disease. J Mol Neurosci 50:600–607
Copeland RL, Das JR, Kanaan YM (2007) Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res 12:61–69
Chao CC, Hu S, Ehrlich L, Peterson PK (1995) Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun 9:355–365
Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Colditz GA, Speizer FE, Ascherio A (2003) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 60:1059–1064
Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK (2013) Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson’s disease? J Intern Med 273:429–436
Das JR, Tizabi Y (2009) Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res 16:194–204
Delgado M, Ganea D (2001) Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol 167:966–975
Dong-Kug C, Kyoungho S (2007) Glial reaction in Parkinson’s diseases: inflammatory activation signaling of slia as a potential therapeutic target. Curr Signal Transduction Therapy 2:77–90
Frechilla D, Garcia-Osta A, Palacios S, Cenarruzabebeitia E, Del Rio J (2001) BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. NeuroReport 12:919–923
Ganea D, Delgado M (2002) Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) as modulators of both innate and adaptive immunity. Crit Rev Oral Biol Med 13:229–237
Gayle DA, Ling Z, Tong C, Landers T, Lipton JW, Carvey PM (2002) Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Brain Res Dev Res 133:27–35
Haanen C, Vermes I (1995) Apoptosis and Inflammation. Mediators Inflamm 4:5–15
Jantas D, Roman A, Kuśmierczyk J, Lorenc-Koci E, Konieczny J, Lenda T, Lasoń W (2013) The extent of neurodegeneration and neuroprotection in two chemical in vitro models related to Parkinson’s disease is critically dependent on cell culture conditions. Neurotox Res 24:41–54
Klegeris A, Bissonnette CJ, McGeer PL (2003) Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 139:775–786
Kuwano K, Hara N (2000) Signal transduction pathways of apoptosis and inflammation induced by the tumor necrosis factor receptor family. Am J Respir Cell Mol Biol 22:147–149
Liu B, Hong JS (2003) Role of Microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharamacol Exp Ther 304:1–7
Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, Brabet P, Leceta J, Gomariz RP (2002) Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA 99:1053–1058
Maruyama W, Yi H, Takahashi T, Shimazu S, Ohde H, Yoneda F, Iwasa K, Naoi M (2004) Neuroprotective function of R-(-)-1-(benzofuran-2-yl)-2-propylaminopentane, [R-(-)-BPAP], against apoptosis induced by N-methyl(R)salsolinol, an endogenous dopaminergic neurotoxin, in human dopaminergic neuroblastoma SH-SY5Y cells. Life Sci 75:107–117
McGeer PL, Yasojima K, McGeer EG (2001) Inflammation in Parkinson’s disease. Adv Neurol 86:83–89
Naoi M, Maruyama W, Nagy GM (2004) Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology 25:193–204
Nakamura Y, Si QS, Kataoka K (1999) Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35:95–100
Ohira H, Fujioka Y, Katagiri C, Yano M, Mamoto R, Aoyama M, Usami M, Ikeda M (2012) Butyrate enhancement of inteleukin-1β production via activation of oxidative stress pathways in lipopolysaccharide-stimulated THP-1 cells. J Clin Biochem Nutr 50:59–66
Qualls Z, Brown, D, Ramlochansingh C, Hurley LL, Tizabi Y (2013) Protective effects of curcumin against rotenone and salsolinol-induced toxicity: implications for Parkinson’s disease. Neurotox Res (E-pub ahead of print)
Racz B, Tamas A, Kiss P, Toth G, Gasz B, Borsiczky B, Ferencz A, Gallyas F Jr, Roth E, Reglodi D (2006) Involvement of ERK and CREB signaling pathways in the protective effect of PACAP in monosodium glutamate-induced retinal lesion. Ann N Y Acad Sci 1070:507–511
Raghavan M, Bjorkman P (1996) Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol 12:181–222
Ramlochansingh C, Taylor RE, Tizabi Y (2011) Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res 20:263–269
Rat D, Schmitt U, Tippmann F, Dewachter I, Theunis C, Wieczerzak E, Postina R, van Leuven F, Fahrenholz F, Kojro E (2011) Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J 25:3208–3218
Reglodi D, Kiss P, Lubics A, Tamas A (2011) Review on the protective effect of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des 17:962–972
Reglodi D, Lubics A, Kiss P, Lengvari I, Gaszner B, Toth G, Hegyi O, Tamas A (2006) Effect of PACAP in 6-OHDA-induced injury of the substantia nigra in intact young and ovariectomized female rats. Neuropeptides 40:265–274
Reglodi D, Tamas A, Lubics A, Szalontay L, Lengvari I (2004) Morphological and functional effects of PACAP in 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regul Pept 123:85–94
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363:1783–1793
Schneider L, Giordano S, Zelickson BR, Johnson MS, Benavides GA, Ouyang X, Fineberg N, Darley-Usmar VM, Zhang J (2011) Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med 51:2007–2017
Schulte T, Schols L, Muller T, Woitalla D, Berger K, Kruger R (2002) Polymorphisms in the interleukin-1 alpha and beta genes and the risk for Parkinson’s disease. Neurosci Lett 326:70–72
Somogyvari-Vigh A, Reglodi D (2004) Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des 10:2861–2889
Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, Matsubara K, Ohta S, Wolf HU, Schwarz J (2002) Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson’s disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol 63:909–920
Szabadfi K, Danyadi B, Kiss P, Tamas A, Fabian E, Gabriel R, Reglodi D (2012) Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J Mol Neurosci 48:501–507
Suk K, Park JH, Lee WH (2004) Neuropeptide PACAP inhibits hypoxic activation of brain microglia: a protective mechanism against microglial neurotoxicity in ischemia. Brain Res 1026:151–156
Theus SA, Cave MD, Eisenach KD (2004) Activated THP-1 cells: an attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect Immun 72:1169–1173
Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K (1980) Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26:171–176
Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H (2000) The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells in mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci USA 97:13390–13395
Vaudry D, Pamantung TF, Basille M, Rousselle C, Fournier A, Vaudry H, Beauvillain JC, Gonzalez BJ (2002) PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci 15:1451–1460
Vaudry D, Falluel-Morel A, Bougault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Vertongen P, Devalck C, Sariban E, De Laet MH, Martelli H, Paraf F, Hélardot P, Robberecht P (1996) Pituitary adenylate cyclase activating peptide and its receptors are expressed in human neuroblastomas. J Cell Physiol 167:36–46
Wang G, Pan J, Tan Y, Sun XK, Zhang YF, Zhou HY, Ren RJ, Wang XJ, Chen SD (2008) Neuroprotective effects of PACAP27 in mice model of Parkinson’s disease involved in the modulation of K (ATP) subunits and D2 receptors in the striatum. Neuropeptides 42:267–276
Watson MB, Nobuta H, Abad C, Lee SK, Bala N, Zhu C, Richter F, Chesselet MF, Waschek JA (2013) PACAP deficiency sensitizes nigrostriatal dopaminergic neurons to paraquat-induced damage and modulates central and peripheral inflammatory activation in mice. Neuroscience 240:277–286
Wersinger C, Sidhu A (2002) Inflammation and Parkinson’s disease. Curr Drug Targets Inflamm Allergy 1:221–242
Yaka R, He D, Phamluong K, Ron D (2003) Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem 278:9630–9638
Yang S, Yang J, Yang Z, Chen P, Fraser A, Zhang W, Pang H, Gao X, Wilson B, Hong JS, Block ML (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther 319:595–603
Acknowledgment
Supported by NIH/NIGMS 2 SO6 GM08016-39 and Howard University College of Medicine Bridge Fund (YT) and KTIA NAP 13-1-2013-0001, MTA Momentum Program, TAMOP4.2.2.A-11/1/KONV-2012-0024), Arimura Foundation, and OTKA K104984 (AT, DR)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, D., Tamas, A., Reglodi, D. et al. PACAP Protects Against Inflammatory-Mediated Toxicity in Dopaminergic SH-SY5Y Cells: Implication for Parkinson’s Disease. Neurotox Res 26, 230–239 (2014). https://doi.org/10.1007/s12640-014-9468-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-014-9468-x