Abstract
Purpose
Nabilone is a synthetic cannabinoid with properties that make it an appealing candidate as a postoperative nausea and vomiting (PONV) prophylactic adjunct. Nabilone has proven clinical utility in chemotherapy-related nausea and vomiting but has not been adequately tested for PONV. The purpose of this study was to evaluate the effectiveness of a single dose of nabilone for the prevention of PONV.
Methods
This was a pragmatic single-centre randomized-controlled trial comparing oral nabilone vs placebo for the prevention of PONV. Eligible patients scheduled for elective surgery under general anesthesia who had a preoperative risk of PONV greater than 60% received either nabilone 0.5 mg or placebo orally prior to surgery. As part of the pragmatic design, the study medication was given in addition to any other combination of antiemetic prophylaxis. The primary outcome was the incidence of PONV. Secondary outcomes included the effect on pain, speed of recovery, and drug side effects.
Results
Of the 340 patients randomized, 172 received nabilone and 168 received placebo. There was no difference in the incidence of PONV, which occurred in 20.9% in the nabilone group and 21.4% in the placebo group (relative risk, 0.98; 95% confidence interval, 0.89 to 1.11; P = 0.99). There were also no differences in pain scores, opioid consumption, or reported drug side effects.
Conclusion
Oral nabilone 0.5 mg given as a single dose prior to surgery is ineffective in reducing PONV. This trial was registered at ClinicalTrials.gov, identifier: NCT02115529.
Résumé
Objectif
Le nabilone est un cannabinoïde de synthèse possédant des propriétés potentiellement intéressantes pour complémenter la prophylaxie de prévention des nausées et vomissements postopératoires (NVPO). Cet agent a une utilité clinique éprouvée pour traiter les nausées et vomissements liés à la chimiothérapie, mais il n’a pas été adéquatement testé pour le traitement des NVPO. L’objectif de cette étude était d’évaluer l’efficacité d’une dose unique de nabilone pour la prévention des NVPO.
Méthode
Nous avons réalisé une étude randomisée contrôlée unicentrique pragmatique afin de comparer le nabilone oral à un placebo pour prévenir les NVPO. Les patients éligibles devant subir une chirurgie non urgente sous anesthésie générale et qui présentaient un risque préopératoire de NVPO supérieur à 60 % ont reçu 0,5 mg de nabilone ou un placebo par voie orale avant leur chirurgie. Dans le cadre de notre méthodologie pragmatique, le médicament à l’étude a été administré en ajout à toute autre combinaison prophylactique antiémétique. Le critère d’évaluation principal était l’incidence de NVPO. Les critères d’évaluation secondaires comprenaient l’effet sur la douleur, la rapidité de récupération, et les effets secondaires du médicament.
Résultats
Parmi les 340 patients randomisés, 172 ont reçu du nabilone et 168 un placebo. Aucune différence n’a été observée dans l’incidence des NVPO, qui sont survenus chez 20,9 % des patients du groupe nabilone et 21,4 % des patients du groupe placebo (risque relatif, 0,98; intervalle de confiance 95 %, 0,89 à 1,11; P = 0,99). Aucune différence n’a été observée non plus en matière de scores de douleur, de consommation d’opioïdes, ou d’effets secondaires rapportés du médicament.
Conclusion
Une dose de 0,5 mg de nabilone administrée oralement en dose unique avant la chirurgie est inefficace pour réduire les NVPO. Cette étude a été enregistrée au ClinicalTrials.gov, identifiant: NCT02115529.
Similar content being viewed by others
Untreated, one-third of patients undergoing general anesthesia will have postoperative nausea, vomiting, or both (PONV),1 and in high-risk patients, up to 80% may experience PONV.1,2 The occurrence of PONV frequently delays discharge and is the leading cause of unexpected hospital admission after planned ambulatory surgery.3 There can also be significant costs associated with the treatment of PONV and their sequelae.4-6 No single intervention is consistently effective for the prevention of PONV, and many of the strategies are either impractical (e.g., avoiding opiates) or have potential risks of serious side effects (e.g., QTc prolongation with 5-HT receptor antagonists7 and antidopaminergic drugs).8
The medical uses of cannabis and cannabinoids are expanding.9 The known pharmacokinetic and pharmacodynamic properties of cannabinoids,10 as well as their previously demonstrated clinical utility for chemotherapy-related nausea and vomiting,11,12 make them an appealing candidate as a prophylactic PONV adjunct. Nabilone (Cesamet®; Valeant Pharmaceuticals North America / MEDA Pharmaceuticals Inc.; Somerset, NJ, USA), a synthetic cannabinoid developed in the 1970s, is a potent cannabinoid receptor type 1 (CB1) agonist.13 Just as there has been previous success in translating treatments for chemotherapy-induced nausea and vomiting (i.e., 5-HT receptor antagonists, including ondansetron and granisetron) into the perioperative environment,14 we were interested in understanding if nabilone could similarly be beneficial in preventing PONV.
Thus, the aim of this study was to determine if preoperative nabilone 0.5 mg is efficacious in preventing PONV. As secondary outcomes, we examined the effect of nabilone on acute postoperative pain and consumption of pain medications, its safety and side-effect profile, as well as whether the effects of the medication changed patient flow through the postanesthesia care unit (PACU).
Methods
Study design
This was a single-centre randomized-controlled trial assessing oral nabilone vs placebo for the prevention of PONV. The Research Ethics Boards of the study centre approved the protocol (November, 2013), and written informed consent was obtained from all patients. As this was an off-label use for nabilone, the Health Canada Therapeutic Product Directorate approved the study protocol and all protocol changes (control number - 163759; first date of approval – June 27, 2013). The recruitment period lasted from July 14, 2014 to January 16, 2015.
Study population
Patients attending the preanesthesia facility (PAF) or admitted to hospital preoperatively in preparation for elective surgery with general anesthesia were screened for eligibility. Eligibility criteria were patients > 18 yr of age, American Society of Anesthesiologists (ASA) physical status I – III,16 able to swallow the study medication, and with at least three of four Apfel risk factors for PONV (i.e., female sex, non-smoker status, anticipated use of postoperative opioid, and previous PONV or motion sickness).2,15 Patients were excluded if postoperative PACU admittance was not anticipated (e.g., scheduled for immediate postoperative transfer to the intensive care unit [ICU]); if they had a known sensitivity to marijuana or other cannabinoid agents, a psychotic illness or depression, or an addiction to illicit substances (including cannabis) or alcohol; if they were pregnant or lactating, suffered from chronic nausea and/or vomiting, or were treated with any other investigational drug within 12 weeks prior to randomization.
Randomization and blinding
An epidemiologist not directly involved in either the execution of the study or the analysis of the study results prepared a computer-generated restricted randomization schedule with a 1:1 allocation ratio and randomly varying block sizes. To maintain allocation concealment, the research pharmacy used sequentially numbered sealed opaque envelopes to assign and prepare the study drug or placebo for each enrolled patient in sequence on the day of surgery.
To ensure blinding, the nabilone and placebo pills were identical in appearance. All study investigators, study coordinators, participants, clinicians, and data analysts were blinded to group allocation for the duration of the study. The research pharmacy maintained a sealed envelope for each enrolled patient. The envelopes were labelled with the subject number and contained information for emergency unblinding if needed.
Study interventions
On the day of surgery, patients were randomized to receive either oral nabilone 0.5 mg or placebo to be taken within three hours of the induction of anesthesia. The single 0.5-mg dose was chosen for several reasons. It minimized the risk of adverse side effects, removed adherence and safety concerns regarding home dosing and the implications around doses taken before delayed or cancelled surgeries, and also offered a practical regimen that could be readily adopted in most perioperative settings. Furthermore, the doses needed for PONV prophylaxis generally tend to be lower than those needed for chemotherapy-related nausea and vomiting.17,18
As this was designed as a pragmatic trial, there were no restrictions placed on the attending anesthesiologist restricting the provision of other antiemetic therapy or prophylaxis—i.e., the study medication was given in addition to any other combination of antiemetic prophylaxis.
Study outcomes
After the patients arrived in the PACU, study outcomes were collected at intervals of 30, 60, 120, 180, 240, and 300 min, or until discharge from the PACU. The primary outcome was the presence of symptoms of nausea or vomiting (i.e., emesis, retching, or heaving) at any time after admission to the PACU. This was measured using a modified PONV impact scale (where 0 = no PONV; 1 = nausea; and 2 = vomiting).19 A score greater than zero at any time point in the PACU was recorded as meeting the primary outcome.
Secondary outcomes included pain, which was examined both with and without movement at each data collection interval using an 11-point numerical rating scale (NRS). An NRS score of 0 = no pain and a score of 10 = extreme pain.20 We also recorded intra- and postoperative morphine equivalent consumption throughout the procedure and in the PACU. We used the institutional opioid conversion chart (Appendix) to convert all opioid medications to a common base unit of morphine 10 mg iv for comparison. The recovery characteristics examined included the PACU length of stay and time to achieve PACU discharge criteria (i.e., a modified Aldrete score21 > 8/10).
Patients’ baseline symptoms were solicited on the day of surgery before they ingested the trial medication (nabilone or placebo). Potential side effects (those reported in the Cesamet monograph) were again solicited from the patients upon exiting the PACU. Changes in symptomatology were reported.
Exploratory analysis
To adjust for possible situations in which PONV occurred and resolved in-between the discrete data collection intervals, a composite measure, which included a PONV score > 0 and/or any antiemetic treatment in the PACU (i.e., dimenhydrinate, ondansetron, metoclopramide, or dexamethasone), was examined. All analyses of the primary outcome were completed and reported using both the simple and composite measures.
Statistical analysis
We estimated that the primary outcome measure would occur in 39% of the control group. This was calculated by estimating a baseline risk of 61-78%2 (based on the presence of at least three Apfel risk factors) and that the attending anesthesiologist would give each patient one to two prophylactic agents (based on the practice patterns at the study centre), thus reducing the new baseline risk to 39%.22 In order to detect a clinically significant reduction of PONV to 25%—i.e., a 35% relative reduction in PONV, similar to the best effect of current antiemetic agents—15,22 a sample size of 165 patients would be needed in each group, assuming a normal distribution in both the control and study groups with an alpha = 0.05 and beta = 0.20. To account for a higher than expected number of patients whose surgery was cancelled preoperatively or who were admitted to the ICU instead of the PACU, the total number of randomized patients was increased to 420 (210 patients in each arm).
The primary intention-to-treat analysis included all patients who received the study drug, underwent the planned surgery, and had available outcomes data. Missing data were not imputed. Baseline characteristics of the patients in the two treatment groups were reported using frequency distributions (%), with descriptive statistics using mean (standard deviation [SD]) or median [interquartile range (IQR)] where appropriate.
The principal analysis of the primary outcome, frequency of PONV, was performed using an unadjusted Chi square test comparing the proportion of events in each treatment group.
For secondary outcomes, continuous variables with normally distributed data, including opioid administration and NRS pain scores, were compared using Student’s t test. Further logistic regression analyses examined the effect of clinically relevant covariates that were known or suspected predictors of the outcome (e.g., number of concurrent antiemetic prophylactic agents, opioid dose, surgery type) or that reflected imbalances at baseline. The types of surgeries that were suspected of increasing the risk of PONV were bariatric, gynecologic, and laparoscopic surgery. An analysis of drug side effects was completed using analysis of covariance to account for the change from baseline. To compute the effect size and significance, we compared the rates between the two groups and tested for difference being zero. The PACU length of stay and time to achieve a suitable recovery score for discharge from the PACU (a modified Aldrete score21 > 8/10) were analyzed using a two-sample Wilcoxon non-parametric test to compare groups.
Ninety-five percent confidence intervals (95% CIs) were calculated for all unadjusted results. Adjusted calculations are reported without 95% CIs. All analyses were conducted using SAS® 9.4 (SAS Institute Inc., Cary, NC, USA). All hypothesis tests were two-tailed with a significance level of 0.05.
Results
Study population
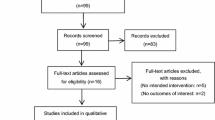
Approximately 7,000 patients attended the PAF during the recruitment period, and 2,000 patients were screened for the presence of at least three Apfel PONV risk factors. Four hundred nine participants met the inclusion criteria and consented to participate in this trial (Figure). Twenty participants withdrew consent prior to randomization. Seven patients had their surgery cancelled prior to randomization, and six patients did not have their operation scheduled before study completion. Sixteen patients were never randomized due to logistical complications on the day of surgery, and 20 patients were removed from the study prior to randomization after a re-evaluation of the inclusion/exclusion criteria on the day of surgery. In total, 340 patients were randomized; 168 patients received a placebo and 172 received nabilone 0.5 mg.
Six patients, all in the placebo group, had missing outcome data. Five patients received the study treatment and subsequently had their surgery cancelled due to unanticipated operating room staffing or scheduling conflicts (i.e., preceding case was longer than expected), and one patient was unexpectedly admitted to the ICU where outcomes data could not be collected (Figure).
Baseline demographics
Baseline demographic and clinical characteristics were similar in both groups (Table 1). Both groups had similar preoperative risks of PONV based on the number of Apfel risk factors. There was a similar interval between ingesting the study drug and entering the operating room. All randomized patients were female, although not by methodological design. A criterion for inclusion in the study was having three of four risk factors for PONV, and since being female counted as a risk factor, most of the patients enrolled in the study were female. A small number of male patients were enrolled; however, by coincidence, all were excluded prior to randomization. There was a similar distribution of types of surgery.
Primary outcome
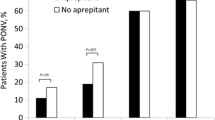
There was no difference in the incidence of PONV between groups (Table 2). Postoperative nausea and vomiting occurred in 36/172 (20.9%) patients in the nabilone group and 36/168 (21.4%) patients in the placebo group (relative risk, 0.98; 95% CI, 0.89 to 1.11; P = 0.99)
Secondary outcomes
There were no differences in pain scores between groups, either at rest or with movement, at the first measurement or regarding the maximum pain score recorded. There were no differences in intra- or postoperative opioid doses between groups (Table 4). The nabilone group achieved a rest and recovery score (RRS) > 8 (i.e., meeting PACU discharge criteria) four minutes earlier than the placebo group (median [IQR] time, 31 [30-40] min vs 35 [30-65] min, respectively; P = 0.025). This did not result in faster discharge from the PACU (Table 2).
Medication side effects
One side effect, i.e., lack of muscle coordination, showed a difference between groups. Preoperatively, 1/172 (0.6%) patients in the nabilone group and 3/168 (1.8%) patients in the placebo group had this symptom. Postoperatively, 3/172 (1.7%) patients in the nabilone group and 0 (0%) patients in the placebo group experienced this symptom (P < 0.001). None of the other symptoms showed a difference between groups (Table 5).
Exploratory analysis
When the use of any antiemetic treatment was included as part of the composite primary outcome measure, the incidence of PONV was 72/172 (41.7%) and 70/168 (41.8%) in the nabilone and placebo groups, respectively (relative risk, 0.99; 95% CI, 0.77 to 1.28; P = 1.00). Furthermore, there was no difference in the rates of vomiting alone (nabilone, 2.3% vs placebo, 1.9%; relative risk, 1.21; 95% CI, 0.30 to 5.73; P = 1.00).
There was no difference in PONV after multivariate adjustment for intra- and postoperative opioid dosing; however, data were insufficient to adjust independently for the intraoperative morphine equivalent dose. There was no difference between groups when adjusting for bariatric, gynecologic, and laparoscopic surgery (Table 3).
Discussion
In this adequately powered pragmatic randomized-controlled trial, nabilone 0.5 mg given as a single oral dose prior to surgery was ineffective in reducing the incidence of acute PONV. The study population represents a heterogeneous group of females with a variety of different surgeries, increasing the generalizability of these results. The known confounders, including surgical type, morphine equivalent dose, duration of anesthesia, and number of prophylactic antiemetics, were well balanced between groups.
In 1995, a previous randomized-controlled trial studied oral nabilone for the reduction of PONV.23 This study compared nabilone 2 mg vs metoclopramide 10 mg before elective hysterectomy in 53 females who received general anesthesia with sodium thiopental and 66% nitrous oxide. This study failed to show any significant difference in the incidence of PONV. There were some limitations to that study, including a poorly optimized dose and dosing regimen, a small sample size, and a comparison group lacking clinical generalizability. Furthermore, the modern anesthetic technique and antiemetic prophylactic treatments have changed considerably. In contrast, one retrospective study showed a marked relative risk reduction of 75% for PONV with a combination of preoperative oral dronabinol and rectal prochlorperazine,24 adding to the impetus for further research. A more recent study examined intravenous delta-9-tetrahydrocannabinol for the prevention of PONV.25 The investigators used the rationale that the intravenous route would be beneficial by avoiding first-pass metabolism by the liver, which may improve drug efficacy. The study was stopped early due to a high rate of unwanted side effects, including increased sedation, confusion, and extreme and sustained mood swings or anxiety without significantly reducing the effects of PONV. Our study did not reveal any issues with increased side effects from the use of nabilone.
There are several possible explanations for lack of efficacy in our trial. The dose may have been too small or the timing may not have been ideal. Four trials that have shown nabilone to be effective in treating chemotherapy-related nausea and vomiting used a higher multi-dose regimen.26-29 Patients received the first dose the night before the chemotherapy and the second and third doses before and after chemotherapy. Also, the drug doses (all used 2-mg doses) were higher than in our study. As previously mentioned, the rational for the lower dose was in keeping with the goals of identifying a pragmatic antiemetic adjunct that could be readily and broadly adopted should this intervention be found effective.
With regard to optimizing the timing of administration, it is important to consider the pharmacokinetics of nabilone. Oral nabilone has a half-life of approximately two hours.30 The mean (SD) time from drug ingestion to arrival in the PACU was 160 (82) min and 168 (81) min in the nabilone and placebo groups, respectively. Therefore, on average, approximately 1.4 drug half-lives had passed by the time the patients were first assessed for PONV. This duration between ingestion and emergence from anesthetic may have resulted in low circulating drug concentrations in the PACU. The clinical significance of this difference is uncertain. Other studies of PONV prophylaxis have shown that the timing of administration can modify their effect. For example, dexamethasone tends to have better efficacy when given early in the anesthesia period, whereas ondansetron is more effective when given close to emergence.31 This leads to questions about the mechanism of action of the various antiemetic drugs. In other words, is the mechanism of action related to receptor modulation during emetic triggering while the patient is under anesthetic or is it related to nausea suppression while the patient is awake and conscious? Multiple-dose trials using different timing of dose might explain this more clearly. Alternatively, the nausea chemoreceptors triggered by cancer therapeutics and then treated by nabilone may not be involved in the pathophysiology of PONV. This may have to do with the unique properties of this particular synthetic cannabinoid or it may be extrapolated to all cannabinoids. Further research on the pathophysiology is required.
As a secondary outcome, we examined the effect of nabilone on acute postoperative pain. The endocannabinoid system is known to regulate the pain perception in humans. Cannabinoids acting at CB1 downregulate nociceptive transmission throughout the nervous system and can theoretically diminish the perception of pain.32 A systematic review of single-dose cannabinoids for the treatment of acute pain found them to be equivalent to codeine and better than placebo, with 16 being the number-needed-to-treat to achieve a 50% reduction in pain.33 The results from our trial showed that nabilone had no impact on acute postoperative pain in the PACU. Although previous studies have detected a pain-reducing effect of cannabinoids, this effect has been small in magnitude, doses of other pain medications were controlled, and pain stimuli were recorded.33 None of these controls were in place in this trial. As regards the effect of nabilone on PONV, changes in dose and timing may ultimately reveal an effect, and further research could answer these questions.
There are several limitations to this study. All of the subjects were female. This may limit the generalizability of our findings—although we speculate that it is unlikely that males would react differently than females to nabilone. In addition, the event rates in our trial were relatively low compared with other studies. When measuring PONV scores greater than 0, PONV occurred at a rate of 21% in both groups (Table 2). Based on known risk factors and prophylactic drug efficacies, the expected rate in the placebo group was 40-49%. Patients in our study had a baseline risk of 61-79%.15 They were treated with an average of 1.6 concurrent PONV prophylactic antiemetics (Table 1), and each antiemetic typically reduces the preoperative risk by 21%.22 Another limitation is our data collection technique for the primary outcome, i.e., measuring only documented subjective sensations of PONV may have underestimated the true event rates. We attempted to compensate for this shortcoming by creating an exploratory composite measure of the primary outcome that included patients who were treated with antiemetics or were symptomatic, and the detected event rate was adjusted to approximately 42%, much closer to the rates predicted in the literature. Future studies would benefit from either a PACU exit question asking if there was any PONV over the course of the PACU stay or a higher frequency of PONV assessments to capture PONV that falls between discreet data collection intervals. Once again, this likely represents a non-differential form of bias, and if the drug had been more effective, at least some signal should have been seen. In this study, both groups had very similar rates of PONV regardless of the measure used to assess them.
Conclusion
In summary, nabilone 0.5 mg given orally as a single dose prior to surgery was not effective in preventing PONV.
References
Gan TJ. Postoperative nausea and vomiting–can it be eliminated? JAMA 2002; 287: 1233-6.
Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999; 91: 693-700.
Fortier J, Chung F, Su J. Unanticipated admission after ambulatory surgery–a prospective study. Can J Anaesth 1998; 45: 612-9.
Dzwonczyk R, Weaver TE, Puente EG, Bergese SD. Postoperative nausea and vomiting prophylaxis from an economic point of view. Am J Ther 2012; 19: 11-5.
Gupta D, Haber H. Emetogenicity-risk procedures in same day surgery center of an academic university hospital in United States: a retrospective cost-audit of postoperative nausea vomiting management. Middle East J Anaesthesiol 2014; 22: 493-502.
Parra-Sanchez I, Abdallah R, You J, et al. A time-motion economic analysis of postoperative nausea and vomiting in ambulatory surgery. Can J Anesth 2012; 59: 366-75.
Doggrell SA, Hancox JC. Cardiac safety concerns for ondansetron, an antiemetic commonly used for nausea linked to cancer treatment and following anaesthesia. Expert Opin Drug Saf 2013; 12: 421-31.
Spevak C, Hamsher C, Brown CQ, Wedam EF, Haigney MC. The clinical significance of QT interval prolongation in anesthesia and pain management: what you should and should not worry about. Pain Med 2012; 13: 1072-80.
Beaulieu P, Boulanger A, Desroches J, Clark AJ. Medical cannabis: considerations for the anesthesiologist and pain physician. Can J Anesth 2016; 63: 608-24.
Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 2014; 722: 134-46.
Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001; 323: 16-21.
Ware MA, Daeninck P, Maida V. A review of nabilone in the treatment of chemotherapy-induced nausea and vomiting. Ther Clin Risk Manag 2008; 4: 99-107.
Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 2010; 17: 1360-81.
Hsu ES. A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am J Ther 2010; 17: 476-86.
Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 2012; 109: 742-53.
Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178: 261-6.
Tricco AC, Soobiah C, Blondal E, et al. Comparative efficacy of serotonin (5-HT3) receptor antagonists in patients undergoing surgery: a systematic review and network meta-analysis. BMC Med 2015; 13: 136.
Jordan K, Hinke A, Grothey A, et al. A meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy-induced emesis. Support Care Cancer 2007; 15: 1023-33.
Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth 2012; 108: 423-9.
Chauny JM, Paquet J, Lavigne G, Marquis M, Daoust R. Evaluating acute pain intensity relief: challenges when using an 11-point numerical rating scale. Pain 2016; 157: 355-60.
Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. Journal of perianesthesia nursing: official journal of the American Society of PeriAnesthesia Nurses / American Society of PeriAnesthesia Nurses 1998; 13: 148-55.
Apfel CC, Korttila K, Abdalla M, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441-51.
Lewis IH, Campbell DN, Barrowcliffe MP. Effect of nabilone on nausea and vomiting after total abdominal hysterectomy. Br J Anaesth 1994; 73: 244-6.
Layeeque R, Siegel E, Kass R, et al. Prevention of nausea and vomiting following breast surgery. Am J Surg 2006; 191: 767-72.
Kleine-Brueggeney M, Greif R, Brenneisen R, Urwyler N, Stueber F, Theiler LG. Intravenous delta-9-tetrahydrocannabinol to prevent postoperative nausea and vomiting: a randomized controlled trial. Anesth Analg 2015; 121: 1157-64.
Einhorn L. Nabilone: an effective antiemetic agent in patients receiving cancer chemotherapy. Cancer Treat Rev 1982; 9 Suppl B: 55-61.
Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev 1982; 9 Suppl B: 45-8.
Levitt M. Nabilone vs. placebo in the treatment of chemotherapy-induced nausea and vomiting in cancer patients. Cancer Treat Rev 1982; 9 Suppl B: 49-53.
Wada JK, Bogdon DL, Gunnell JC, Hum GJ, Gota CH, Rieth TE. Double-blind, randomized, crossover trial of nabilone vs. placebo in cancer chemotherapy. Cancer Treat Rev 1982; 9 Suppl B: 39-44.
Valeant Canada Limitée/Limited. Product monograph, Nabilone; submission control no: 124406. 2009. Available from URL: http://www.editorialmanager.com/caan/download.aspx?id=90945&guid={A1DB26F5-FE4C-49F7-AA37-99FB10C2E271}&scheme=1 (accessed November 2016).
Wang XX, Zhou Q, Pan DB, et al. Dexamethasone versus ondansetron in the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2015; 15: 118.
Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Hand Exp Pharmacol 2015; 227: 119-43.
Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 2001; 323: 13-6.
Acknowledgments
This trial was supported in part by a Physicians’ Services Incorporated Resident’s Research Grant. Valeant Canada donated the study medications and placebo. Neither Physicians’ Services Incorporated nor Valeant Canada had any role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
David Levin, An-Wen Chan, C. David Mazer, and Aaron Hong were involved in the study design. David Levin and Zachary Dulberg were involved in study execution. David Levin, Zachary Dulberg, An-Wen Chan, Greg Hare, C. David Mazer, and Aaron Hong were involved in statistical analysis. David Levin wrote the primary draft of the manuscript. An-Wen Chan, Greg Hare, C. David Mazer, and Aaron Hong were involved in manuscript development. Greg Hare was involved in the acquisition of data. Aaron Hong was involved in study conception.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Institutional opioid conversion table
Rights and permissions
About this article
Cite this article
Levin, D.N., Dulberg, Z., Chan, AW. et al. A randomized-controlled trial of nabilone for the prevention of acute postoperative nausea and vomiting in elective surgery. Can J Anesth/J Can Anesth 64, 385–395 (2017). https://doi.org/10.1007/s12630-017-0814-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0814-3