Abstract
Purpose
We investigated the effects of tidal volume (VT), fresh gas flow (FGF), and a charcoal filter in the inspiratory limb on the washout of sevoflurane from the following Datex Ohmeda® (GE) Anesthesia Workstations (AWSs): Aisys®, Aestiva®/5, and Excel 210SE.
Methods
After equilibrating the AWSs with 2% sevoflurane, the anesthetic was discontinued, and the absorbent anesthesia breathing circuit (ABC), reservoir bag, and test lung were changed. The lung was ventilated with 350 or 200 mL·breath−1, 15 breaths·min−1, and a FGF of 10 L·min−1 while the washout of sevoflurane was performed in triplicate using a calibrated Datex Ohmeda Capnomac Ultima™ and a calibrated MIRAN SapphIRe XL ambient air analyzer until the concentration was ≤ 10 parts per million (ppm). The effects of decreasing the FGF to 5 and 2 L·min−1 after the initial washout and of a charcoal filter in the ABC were recorded separately.
Results
The median washout times with the Aisys AWS (14 min, P < 0.01) and the Aestiva/5 (17 min, P < 0.001) with VT 350 mL·breath−1 were significantly less than that with the Excel 210SE (32 min). The mean (95% confidence interval) washout time with the Aisys increased to 23.5 (21.5 to 25.5) min with VT 200 mL·breath−1 (P < 0.01). Decreasing the FGF from 10 to 5 and 2 L·min−1 with the Aisys caused a rebound in sevoflurane concentration to ≥ 50 ppm. Placement of a charcoal filter in the inspiratory limb reduced the sevoflurane concentration to < 2 ppm in the Aisys and Aestiva/5 AWSs within two minutes.
Conclusion
The GE AWSs should be purged with large FGFs and VTs ~350 mL·breath−1 for ~25 min to achieve 10 ppm sevoflurane. The FGF should be maintained to avoid a rebound in anesthetic concentration. Charcoal filters rapidly decrease the anesthetic concentration to < 2 ppm.
Résumé
Objectif
Nous avons étudié les effets du volume courant (VC), du débit de gaz frais (DGF) et d’un filtre à charbon sur la branche inspiratoire sur l’élimination du sévoflurane des stations de travail d’anesthésie (STA) suivantes de Datex Ohmeda® (GE): Aisys®, Aestiva®/5, et Excel 210SE.
Méthodes
Après avoir équilibré les STA avec du sevoflurane à 2 %, l’anesthésique a été arrêté et le circuit respiratoire d’anesthésie (CRA) absorbant (CRA), le sac réservoir et le poumon test ont été remplacés. Le poumon était ventilé avec 350 ou 200 mL· respiration−1, 15 respirations·min−1, et d’un DGF de 10 L·min−1 tandis que l’élimination du sévoflurane était assurée en triple au moyen d’un DatexOhmeda Ohmeda Capnomac Ultima™ calibré et d’un analyseur d’air ambiant calibré MIRAN saphir XL jusqu’à ce que la concentration soit ≤ 10 parties par million (ppm). Les effets de la baisse du DGF à 5 et 2 L·min−1 après l’élimination initiale, et d’un filtre à charbon dans le CRA ont été enregistrésconsignés séparément.
Résultats
Les temps médians d’élimination avec l’Aisys AWS (14 min, P < 0,01) et l’Aestiva/5 (17 min, P < 0,001) avec un VC de 350 mL· respiration−1 ont été significativement inférieurs à ceux de l’Excel 210SE (32 min). Le temps moyen d’élimination (intervalle de confiance à 95 %) avec l’Aisys a augmenté jusqu’à 23,5 (21,5 à 25,5) minutes avec un VC de 200 mL· respiration−1 (P < 0,01). La réduction du DGF de 10 à 5 et 2 L·min−1 avec l’Aisys a provoqué un rebond de la concentration de sévoflurane à ≥ 50 ppm. Le placement du filtre à charbon dans la branche inspiratoire a abaissé la concentration de sévoflurane à < 2 ppm dans les STA Aisys et Aestiva/5 dans un délai de deux minutes.
Conclusion
Les STA de GE doivent être purgées avec de grands DGF et des VT d’environ 350 mL·respiration−1 pendant environ 25 minutes pour atteindre une concentration de sévoflurane de 10 ppm. Le DGF doit être maintenu pour éviter un rebond de la concentration de l’agent anesthésique. Les filtres à charbon de bois abaissent rapidement la concentration de l’anesthésique à < 2 ppm.
Similar content being viewed by others
Reports have been published regarding washout of inhalational anesthetics from the Datex Ohmeda® (GE) Excel 210SE and S/5 Avance® as well as preliminary data for washout from the Aestiva®/5 in preparation for using these GE anesthesia workstations (AWSs) with malignant hyperthermia susceptible (MHS) patients.1,2 In the case of the Aestiva/5, the washout times for sevoflurane range from 9-25 min.3 The washout of sevoflurane from the GE Aisys® has only recently been investigated.4 That study reported similar washout times from the Aestiva/5 and Aisys using a 500 mL·breath−1 tidal volume (VT) but did not explore the effects of VT, fresh gas flow (FGF), or the presence of charcoal filters. Accordingly, we sought to address these issues to define the preparation of the GE AWSs for MHS patients.
The primary aim of this study was to determine the washout of sevoflurane from the Aisys AWS and to compare the results with those of the Aestiva/5 and the Excel 210SE AWSs. Secondary aims were to define the effects of changes in VT and FGF on the washout of sevoflurane from the three AWSs and the time to washout sevoflurane after the FGF was reduced. In addition, we sought to verify the effects of the Vapor-Clean activated charcoal filters (Dynasthetics LLC, Salt Lake City, UT, USA) on the washout of sevoflurane from the GE AWSs.
Methods
Three GE AWSs, an Aisys, an Aestiva/5, and an Excel 210SE, all of which had been in clinical use for more than one year, were isolated for this study. Before each study, the pre-use AWS checks recommended by the American Society of Anesthesiologists and the manufacturer were completed for each machine.Footnote 1
Equilibration period
For each experiment, 2% sevoflurane in 100% oxygen at 2 L·min−1 was equilibrated in the AWS through a pediatric circle anesthesia breathing circuit (ABC) (Vital Signs, Littlehampton, West Sussex, UK) with mechanical ventilation (for parameters see below) and a 1-L artificial lung (Maquet, Rastatt, Germany) for two hours. Throughout the entire experiment, the artificial lung was ventilated at a respiratory rate of 15 breaths·min−1, an inspiratory:expiratory (I:E) ratio of 1:2, VT either 350 or 200 mL·breath−1, and zero positive end-expiratory pressure above the native pressure. The VT was selected based on the typical VT for a child weighing 20-40 kg and was limited by the maximum internal circuit pressure within the tolerance of the MIRAN SapphIRe XL ambient air analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
Washout period
At the end of the two hours, sevoflurane was discontinued and the ventilator was paused. The reservoir bag, ABC, tubing connections to the MIRAN analyzer, soda lime canister (soda lime was not changed if the charcoal filter was used), and the test lung were all exchanged for clean products within one minute. The FGF was set to 10 L·min−1, and the new test lung was ventilated at a respiratory rate of 15 breaths·min−1, VT either 350 or 200 mL·breath−1, I:E ratio of 1:2, zero positive end-expiratory pressure, and a FGF of 10 L·min−1 unless otherwise stated. All measurements were recorded until the sevoflurane concentration was ≤ 10 parts per million (ppm) or the concentration had stabilized for ~40 min. Four clinically relevant scenarios were investigated, and each experiment was performed in triplicate as summarized in the flowchart (Fig. 1).
Flowchart of the experiments conducted for each anesthesia workstation (AWS) and tidal volume investigated. All washouts were performed in triplicate unless indicated otherwise. Each rectangle represents the washout of sevoflurane at the designated fresh gas flow rate (e.g., 10 L·min−1). Where two or more rectangles are separated by an arrow, the second washout followed the first after the first washout reached 10 parts per million (See Methods for further details)
Effect of tidal volume
The washout profiles of sevoflurane from the Aisys, Aestiva/5, and the Excel 210SE AWSs were determined with VTs of 350 or 200 mL·breath−1. The times to reach 10 ppm with all three AWSs for both VTs were recorded. The times to reach 5 ppm were extrapolated from the terminal washouts using least squares linear regression.
Effect of decreasing the FGF from 10 to 5 L·min−1
The washout in this experiment followed the same protocol as the effect of VT on the washout (above), except that only a VT of 350 mL·breath−1 was used. In this experiment, when the sevoflurane concentration reached ≤ 10 ppm, the FGF was reduced from 10 to 5 L·min−1. The sevoflurane concentration was measured continuously as it rebounded and then decreased again to ≤ 10 ppm or for a total of 40 min after the rebound peaked, whichever came first. The peak sevoflurane concentration during the rebound was recorded. In addition, the time for the sevoflurane concentration to washout after the rebound to ≤ 10 ppm was either measured or, if it failed to reach 10 ppm by 40 min, estimated using least squares regression analysis of the washout profiles after the maximum rebound concentration. The times to reach 5 ppm were extrapolated from the terminal washouts using linear regression.
Effect of decreasing the FGF from 10 to 2 L·min−1
The washout in this experiment followed the same protocol as in the effect of VT on the washout (above) except that only the Aisys AWS with a VT of 200 mL·breath−1 was used. In this experiment, when the washout reached ≤ 10 ppm, the FGF was reduced to 2 L·min−1, and the sevoflurane concentration was recorded until it reached ≤ 10 ppm once again. A relationship was sought between the maximum sevoflurane concentration during the rebound and the FGF used.
Effect of decreasing the FGF to 5 and then 2 L·min−1
The washout in this experiment followed the same protocol as in the effect of VT on the washout (above) except that only the Aisys AWS with a VT of 200 mL·breath−1 was used. In this experiment, when the concentration of sevoflurane reached ≤ 10 ppm, the FGF was decreased to 5 L·min−1. When the sevoflurane concentration peaked and decreased to ≤ 10 ppm, the FGF was decreased to 2 L·min−1 and the sevoflurane concentration was recorded until it peaked again and returned to ≤ 10 ppm.
Effect of inserting a charcoal filter
A charcoal filter was inserted into the inspiratory limb of the Aisys and Aestiva/5 AWSs after the equilibration period. Using a VT of 350 mL·breath−1 and 10 L·min−1 FGF, the sevoflurane concentration was measured using the MIRAN analyzer until it was maintained at < 2 ppm for at least three minutes, at which time the charcoal filter was removed from the ABC. The sevoflurane concentration was measured until it peaked, at which time the charcoal filter was reinserted into the ABC and the sevoflurane concentration was measured once again until it decreased to < 2 ppm. At that time, the FGF was reduced to 2 L·min−1. Once the sevoflurane concentration was consistently < 2 ppm for at least six minutes, the charcoal filter was removed from the ABC (in a single experiment with the Aestiva/5 AWS). The sevoflurane concentration was measured until it reached its maximum value, at which time the charcoal filter was reinserted into the ABC.
Sevoflurane analysis instrumentation
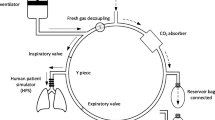
The concentration of sevoflurane within the ABC was analyzed using the Datex Ohmeda Capnomac Ultima™ (Datex Medical Instrumentation Inc., Tewsbury, MA, USA) for inspired concentrations of 2-0.3%, using both the Capnomac and MIRAN analyzers for concentrations of 0.3-0.1%, and using the MIRAN alone for concentrations < 100 ppm. The configuration of the ABC and analyzers is illustrated in Fig. 2.
The Capnomac Ultima was calibrated before each use. Gas for analysis by the Capnomac was aspirated at the elbow of the ABC. The rise time for the Capnomac Ultima was < 520 msec with a measurement range of 0-8% and an accuracy of < 0.2 vol%.
The MIRAN analyzer is a portable infrared spectrophotometer capable of analyzing trace concentrations of inhalational anesthetics, including sevoflurane, to 0.7 ppm in the short path length with accuracy of ± 10% and a sensitivity of 0.1 ppm. The gas analyzer was zeroed before each use. Measurements were recorded until the sevoflurane concentration was < 10 ppm or until a steady-state concentration was reached for approximately 40 min.
The size of the analyzing chamber in the MIRAN analyzer, 2.25 L, requires 3-4 volumetric washouts for an accurate measurement of the anesthetic concentration at small concentrations. Gas for the MIRAN analyzer is aspirated continuously from the inspiratory limb of the ABC at a rate of 14 L·min−1 and returned to the circuit just distal to the expiratory valve, creating a closed loop (Fig. 2). By fashioning the MIRAN within a closed loop in the ABC, the mass balance of sevoflurane within the ABC was preserved to mimic the clinical washout of sevoflurane from the GE AWSs. Smaller aspiration rates would prolong the time to equilibration and thus reduce the frequency of readings. The MIRAN readings were recorded every 30 sec.
All connections within the anesthetic circuit were hand tightened to prevent the loss of gas. The maximum VT was set to 350 mL·breath−1 (or 5.25 L·min−1 minute ventilation at 15 breaths·min−1) to avoid exceeding the maximum allowable pressure of 110 kPa (822.3 mmHg) within the internal gas chamber. To assess the effects of VT on the washout of sevoflurane, a VT of 200 mL·breath−1 (or 3 L·min−1 minute ventilation at 15 breaths·min−1) was also studied.
Statistical analysis
The primary outcome of the study was the washout times to ≤ 10 ppm sevoflurane at a 10 L·min−1 FGF using the Aisys, Aestiva/5, and Excel 210SE AWSs at respective VTs. The washout times for the two VTs for each AWS were compared using the Mann-Whitney U test. If the washout times differed significantly, they were analyzed separately, but if they did not differ, they were grouped together for the analysis of the washout times. The washout times to ≤ 10 ppm were compared among the AWSs using the Kruskal-Wallis test with Dunn’s test to adjust for multiple comparisons. The washout curves for both VTs, 350 and 200 mL·breath−1, were fitted using a two-compartment exponential model and the coefficient of determination (r2) was reported. The times to reach ≤ 10 ppm sevoflurane during the primary washout with 10 L·min−1 FGF and after the rebound with 5 L·min−1 FGF were compared among the AWSs, combining the measurements from all of the experiments. The effect of FGF on the peak concentration of sevoflurane during the rebound period was regressed using a best-fit linear regression approach and the r2 value. Data are presented as raw data or means (95% confidence interval). All tests were two tailed. All reported P values are two-sided.
Results
Effect of tidal volume
The concentration of sevoflurane in the inspiratory limb of the ABC decreased exponentially during the washout phase with all three AWSs at VTs of 350 mL·breath−1 (Fig. 3, upper panel) and 200 mL·breath−1 (Fig. 3, lower panel). The times to ≤ 10 ppm sevoflurane with the Aisys AWS at a VT of 350 mL·breath−1 (P < 0.05) and the Aestiva/5 at a VT of 200 mL·breath−1 (P < 0.05) were significantly less than those with the Excel 210SE at their respective VTs (Table). The washout times for the Aestiva/5 and Excel 210SE AWSs with VTs of 350 and 200 mL·breath−1 were similar and therefore combined (Table). The times for the Aisys at the two VTs differed significantly (Table). When the washout times for the Aisys at 350 and 200 mL·breath−1 and the combined values for the Aestiva/5 and Excel 210SE AWSs (Table) were compared, the times for the Aisys 350 mL·breath−1 (P < 0.001) and for the Aestiva/5 (P < 0.01) were significantly less than that for the Excel 210SE. The estimated times to reach 5 ppm were approximately three-fold greater than the times to reach ≤ 10 ppm (Table).
Composite graphs of the sevoflurane concentrations (parts per million) during washout from the Aisys®, Aestiva®/5, and Excel 210SE anesthesia workstation with a 10 L·min−1 fresh gas flow and VT of 350 mL·breath−1 in the upper panel and VT of 200 mL·breath−1 in the lower panel. Data graphed are mean (SD) (in lieu of raw data for ease of interpretation). For all curves, r2 was ≥ 0.99. VT = tidal volume; ppm = parts per million
Effect of decreasing the FGF from 10 to 5 L·min−1
The sevoflurane concentration rebounded after the FGF was decreased from 10 to 5 L·min−1, reaching secondary peak concentrations that followed the order: Aisys > Aestiva/5 > Excel 210SE (P < 0.0036) (Fig. 4 and Table). The maximum sevoflurane concentration in the Aisys was significantly greater than that in the Excel 210SE (P < 0.05) (Table).
The washout of sevoflurane from three GE anesthesia workstations (using a FGF of 10 L·min−1 and a VT of 350 mL·breath−1) to ≤ 10 ppm that was followed by a reduction in the FGF to 5 L·min−1. Data are mean (SD) (in lieu of raw data for ease of interpretation). Note the magnitude of the rebound in sevoflurane concentration. FGF = fresh gas flow; ppm = parts per million
The times to reach ≤ 10 ppm sevoflurane after the rebound were 63.1 min for the Aisys (r2 = 0.87), 60 min for the Aestiva/5 (r2 = 0.97), and 28.5 min (measured) for the Excel 210SE. The time for the Excel 210SE washout after the rebound is consistent with that estimated by the regression (r2 = 0.96), 29 min. The extrapolated times to reach 5 ppm following the rebound were 73.3 min for the Aisys, 73.8 min for the Aestiva/5, and 49 min for the Excel 210SE.
Effect of decreasing the FGF from 10 to 2 L·min−1
When the FGF was decreased to 2 L·min−1, the sevoflurane concentration rebounded to 70.7 ppm. There was a direct inverse linear relationship between the maximum rebound concentration of sevoflurane and the FGF with the Aisys AWS (r2 = 0.99) (Fig. 5).
Effect of decreasing the FGF to 5 and then 2 L·min−1
The times to rebound and recover to 10 ppm after decreasing the FGF were so protracted that these experiments were prematurely truncated and the data were salvaged only for the washout times to < 10 ppm with a FGF of 10 L·min−1.
Effect of a charcoal filter
With a charcoal filter, the sevoflurane concentration decreased to < 2 ppm within two minutes with both the Aisys and the Aestiva/5 AWSs (at a VT of 350 mL·breath−1 at 15 breaths·min−1) (Fig. 6). After three minutes, the filter was removed and the sevoflurane concentration rebounded. When the sevoflurane concentration reached its peak, the charcoal filter was reinserted into the ABC and the concentration decreased to < 2 ppm. The concentration remained unchanged despite decreasing the FGF to 2 L·min−1. When the filter was removed from the circuit of the Aestiva/5, the sevoflurane concentration rebounded to 248 ppm but abated once the filter was reinserted into the circuit (Fig. 6).
Effects of inserting and removing a charcoal filter from the inspiratory limb of the anesthesia breathing circuit in the Aestiva®/5 anesthesia workstation with a tidal volume of 350 mL·breath−1. The sevoflurane concentration rebounded dramatically to 248 parts per million after the filter was removed at a FGF of 2 L·min−1. FGF = fresh gas flow
Discussion
Our results confirm that the times to sevoflurane washout to ≤ 10 ppm in the Aisys and Aestiva/5 AWSs were significantly less than those with the older Excel 210SE. We determined that VT significantly affects the washout from the Aisys but not from the Aestiva/5. Moreover, when the FGF was reduced from 10 to 5 and 2 L·min−1, the sevoflurane concentration rebounded towards 70 ppm with all three AWSs and returned to ≤ 10 ppm after ~ one hour. With the Aisys, the peak rebound concentration of sevoflurane increased linearly as the FGF decreased, and the time to washout after the rebound was threefold greater than the primary washout. We also demonstrated that a charcoal filter in the inspiratory limb of the ABC rapidly decreased the sevoflurane concentration to < 2 ppm.
Our finding that VT significantly affected the washout times for sevoflurane in the Aisys AWS (Table) is interesting. We posit two explanations for these findings. First, the choice of minute ventilation in this study may be near the lower end that is needed for efficient washout of anesthetic vapour from the breathing circuit. Despite a FGF of 10 L·min−1, a minute ventilation of 3 L·min−1 (VT 200 mL·breath−1 with 15 beats·min−1) significantly prolonged the washout of sevoflurane compared with a 5.25 L·min−1 minute ventilation (Table). We speculate that the slower washout with the VT of 200 mL·breath−1 results from the slower clearance of sevoflurane from the ventilation circuit (including the bellows), the flow sensor, and pressure lines and other pockets within the AWS that trap anesthetic vapour. It should be emphasized that the present study used a FGF and minute ventilations that were less than those recommended by GE Healthcare, i.e., 15 L·min−1 FGF with an 8.4 L·min−1 minute ventilation (VT of 700 mL·breath−1 at a rate of 12 breaths·min−1). Second, the vaporizer design in the Aisys includes the Aladin™ cassette, which interfaces with an electronic vaporizer controller. This controller contains a small quantity of saturated anesthetic vapour (in this case, sevoflurane) trapped in the vaporizer controller that may be injected into the fresh gas flow via a Venturi effect created by the FGF, even after the cassette has been removed from the AWS (Aisys Anesthesia Machine Technical Reference Manual, Version 04/10 M1046983 Page 11-4).
Contaminated AWSs must be purged with large FGFs before they are deemed safe for MHS patients. Studies of the washout of anesthetics from AWSs have targeted concentrations from 4-10 ppm, although the actual concentration that triggers an MH reaction has never been established.5 With the large number of factors that affect the washout, including the type of AWS, FGF, and VT, the washout times among AWSs differ substantively. Moreover, anesthetic analyzers in AWSs cannot measure concentrations in the ppm range. Accordingly, the most prudent strategy is to purge the AWS according to published guidelines to prepare for MHS patients and then to insert a charcoal filter into the inspiratory limb of the ABC.
Reducing the FGF after reaching the target anesthetic concentration causes a rebound in the anesthetic concentration that is severalfold greater than the target concentration (Figs. 4 and 5). We found that decreasing the FGF to 5 L·min−1 caused a rebound in the sevoflurane concentration to seven-fold greater than the 10 ppm target concentration. When the FGF was reduced to 2 L·min−1, the peak was even greater (Figs. 5 and 6). A similar rebound has been reported previously during the washout of GE and other AWSs.4,6 We attribute the rebound in sevoflurane concentration to two causes. First, sevoflurane may have been released from the internal chamber of the AWS, which includes the ventilator bellows and carbon dioxide absorbent canister and their connections located near the FGF common outlet and adjacent to the check valve of the AWSs. Additionally, small pockets of gases containing sevoflurane as well as absorbable rubber components within the AWS may have released anesthetic that appeared more concentrated in the presence of the smaller FGFs. The rebound in sevoflurane concentration can place the patient at risk for MH, and furthermore, reports are lacking regarding the one-hour secondary washout to 10 ppm with the GE Aisys and Aestiva/5 AWSs. Interestingly, no MH reactions have been reported despite the widespread practice of substantially reducing FGFs during anesthesia compared with those used during purging of the AWSs. Accordingly, we strongly recommend that the higher FGF (e.g., 10 L·min−1) used during the washout of the AWS be continued throughout the anesthetic and that a charcoal filter be inserted into the inspiratory limb of the ABC.
In the present study, the 17-min sevoflurane washout time from the Aestiva/5 with a large VT (350 mL·breath−1) is consistent with ~25 min reported recently2 but 100% greater than the time reported in a preliminary study.1 The preliminary study reported a much more rapid washout time for sevoflurane despite using a similar minute ventilation as that in the present study. We suspect that the rapid washout in the preliminary study may be attributed in part to a failure to maintain a mass balance. Since the MIRAN analyzer removes 14 L·min−1 of gas from the anesthetic circuit, the exhaust from the analyzer must be returned to the expiratory limb of the ABC to maintain a mass balance and to avoid dumping sevoflurane from the circuit more rapidly than would be achieved simply by purging with a large FGF.
Several design issues may limit the external validity of this study. First, we limited the maximum VT during mechanical ventilation to ensure the peak inspiratory pressure within the ABC and MIRAN analyzer did not exceed the maximum allowable pressure for the analyzer. If a larger VT (and minute ventilation) had been used with the Aisys, then the washout of sevoflurane would likely have been more rapid. Second, the fact that these AWSs were in clinical service for many years introduces a degree of variability in our measurements similar to that in other studies4 that could limit the external validity of these data. Nevertheless, the coefficients of variation of the sevoflurane concentrations and the washout times (to < 10 ppm) were small among the scenarios, 5-10% for most of the data, and consistent with laboratory standards. Third, we targeted a sevoflurane concentration of 10 ppm to be consistent with our previous study,6 although others have studied the washout to ≤ 5 ppm.4,7-9 In any case, the minimum anesthetic concentration that triggers an MH reaction in either animals or humans has not been established although 1500 ppm halothane, not 1000 ppm, triggered MH reactions in pigs.5 Fourth, we studied only sevoflurane. The washout profiles of other inhalational anesthetics under similar experimental conditions merit investigation because they may differ from that for sevoflurane.1,8
In summary, the washout of sevoflurane from the Aisys AWS depends on the VT and the FGF; a larger VT is preferable during the washout period. A 10 L·min−1 FGF reduces the sevoflurane concentration to < 10 ppm in the Aisys and the Aestiva/5 AWSs within 25 min. The FGF that is used during the initial washout should be maintained to minimize the risk of a rebound in the anesthetic concentration. We recommend the use of a charcoal filter in the inspiratory limb of the AWS when preparing for an MHS patient to ensure the lowest possible anesthetic concentration in the ABC.
Notes
References
Whitaker EE, Kim T. Preparation of GE-Healthcare Aestiva® anesthesia workstations for use with malignant hyperthermia-susceptible patients. Anesth Analg 2011; 112: S-214.
Birgenheier N, Stoker R, Westenskow D, et al. Activated charcoal effectively removes inhaled anesthetics from modern anesthesia machines. Anesth Analg 2011; 112: 1363-70.
Sabouri AS, Lerman J, Heard C. Residual sevoflurane may be present after flushing the GE Anesthesia Workstation for MH Susceptible patients. Anesthesiology 2011; 115: A1276.
Jones C, Bennett K, Kim TW, et al. Preparation of Datex-Ohmeda Aestiva® and Aisys® anaesthetic machines for use in malignant hyperthermia susceptible patients. Anaesth Intens Care 2012; 40: 490-7.
Schutte JK, Becker S, Burmester S, et al. Comparison of the therapeutic effectiveness of a dantrolene sodium solution and a novel nanocrystalline suspension of dantrolene sodium in malignant hyperthermia normal and susceptible pigs. Eur J Anaesthesiol 2011; 28: 256-82.
Petroz GC, Lerman J. Preparation of the Siemens KION anesthetic machine for patients susceptible to malignant hyperthermia. Anesthesiology 2002; 96: 941-6.
Shinkaruk KS, Nolan K, Crossan M. Preparation of the Datex-Ohmeda Aestiva anesthetic machine for malignant hyperthermia cases. Anesthesiology 2008; 109: A279.
Kim TW, Tham RQ. Washout times of desflurane, sevoflurane and isoflurane from the GE healthcare Aisys® and Avance® Carestation® and Aestiva® Anesthesia System. Pediatr Anesth 2013; 23: 1124-30.
Gunter JB, Ball J, Than-Win S. Preparation of the Drager Fabius anesthesia machine for malignant-hyperthermia susceptible patient. Anesth Analg 2008; 107: 1936-45.
Funding
This study was supported solely by departmental funds.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was presented previously, in part, at the annual meeting of the American Society of Anesthesiologists, Chicago, IL, 2011.
Author contributions
A. Sassan Sabouri, Jerrold Lerman, and Christopher Heard helped design the study, conduct the study, and write the manuscript. They have seen the original study data and reviewed the analysis of the data. A. Sassan Sabouri and Jerrold Lerman helped analyze the data. Jerrold Lerman is the author responsible for archiving the study files.
Rights and permissions
About this article
Cite this article
Sabouri, A.S., Lerman, J. & Heard, C. Effects of fresh gas flow, tidal volume, and charcoal filters on the washout of sevoflurane from the Datex Ohmeda® (GE) Aisys®, Aestiva®/5, and Excel 210 SE Anesthesia Workstations. Can J Anesth/J Can Anesth 61, 935–942 (2014). https://doi.org/10.1007/s12630-014-0200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0200-3