Abstract
Purpose
Epidural/spinal opioids are increasingly used to relieve parturients’ pain in labour. Some studies indicate that opioids can induce side effects in neonates, such as respiratory depression and neurobehavioural changes. This meta-analysis aimed to clarify the effects of opioids in labour analgesia on neonates.
Source
PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE™ were searched for relevant randomized controlled trials (RCTs). The neonatal data of Apgar scores, Neurological and Adaptive Capacity Scores (NACS), and umbilical cord pH values were extracted. Statistical analyses were carried out using Review Manager 5.2 and Stata® 10.
Principal findings
Twenty-one trials with 2,859 participants were included in our meta-analysis. No difference in the incidence of Apgar scores < 7 was shown between the opioid and control groups at one minute (risk difference [RD] 0.0%, 95% confidence interval [CI]: −3.0 to 2.0, P = 0.78; I 2 = 0%, 95% CI: 0 to 50) and at five minutes (RD −1.0%, 95% CI: −2.0 to 1.0, P = 0.31; I 2 = 0%, 95% CI: 0 to 50). No significant differences were found in the NACS at two hours (mean difference [MD] −0.35, 95% CI: −1.70 to 1.01, P = 0.62; I 2 = 0%, 95% CI: 0 to 79) and at 24 hr (MD −0.45, 95% CI: −1.36 to 0.46, P = 0.33; I 2 = 3%, 95% CI: 0 to 26). Also, no significant differences were found in umbilical cord artery pH (MD −0.02, 95% CI: −0.06 to 0.03, P = 0.48; I 2 = 80%, 95% CI: 46 to 92) and vein pH (MD −0.03, 95% CI: −0.07 to 0.00, P = 0.08; I 2 = 77%, 95% CI: 36 to 91). No significant publication bias was found.
Conclusion
The common doses of fentanyl and sufentanil used with an epidural/spinal technique in labour analgesia are safe for neonates up to 24 hr after delivery. In future studies, more attention should be paid to the long-term side effects in neonates.
Résumé
Objectif
Les opioïdes administrés par péridurale/rachi sont de plus en plus souvent utilisés pour soulager la douleur du travail chez les parturientes. Quelques études indiquent que les opioïdes peuvent induire des effets indésirables chez les nouveau-nés, tels qu’une dépression respiratoire et des changements neurocomportementaux. Cette méta-analyse visait à clarifier les effets des opioïdes administrés pour l’analgésie du travail sur les nouveau-nés.
Source
Les essais cliniques randomisés pertinents ont été recherchés dans les bases de données Cochrane Central Register of Controlled Trials (CENTRAL) et EMBASE™. Les données néonatales des scores d’Apgar, les scores NACS (Neurological and Adaptive Capacity Scores) et les valeurs du pH du cordon ombilical en ont été extraits. Les analyses statistiques ont été réalisées à l’aide de Review Manager v.5.2 et de Stata® 10.
Constatations principales
Vingt et une études regroupant 2 859 participantes ont été incluses dans notre méta-analyse. Aucune différence dans l’incidence des scores d’Apgar < 7 n’a été montrée entre le groupe opioïdes et le groupe contrôle à une minute (différence de risque [DR] 0,0 %, intervalle de confiance [IC] à 95 %: −3,0 à 2,0, P = 0,78; I 2 = 0 %, IC à 95 %: 0 à 50) et à cinq minutes (DR −1,0 %, IC à 95 %: −2,0 à 1,0, P = 0,31; I 2 = 0 %, IC à 95 %: 0 à 50). Il n’y a pas eu de différence significative pour le NACS à 2 heures (différence moyenne [DM] −0,35, IC à 95 %: −1,70 à 1,01, P = 0,62; I 2 = 0 %, IC à 95 %: 0 à 79) et à 24 h (DM −0,45, IC à 95 %: −1,36 à 0,46, P = 0,33; I 2 = 3 %, IC à 95 %: 0 à 26). De même, aucune différence significative n’a été trouvée pour le pH artériel du cordon ombilical (DM −0,02, IC à 95 %: −0,06 à 0,03, P = 0,48; I 2 = 80 %, IC à 95 %: 46 à 92) et pour le pH veineux (DM −0,03, IC à 95 %: −0,07 à 0,00, P = 0,08; I 2 = 77 %, IC à 95 %: 36 à 91). Aucun biais significatif de publication n’a été identifié.
Conclusion
Les doses habituelles de fentalyl et sufentanil utilisées dans les techniques péridurales/rachi pour l’analgésie du travail sont sécuritaires pour les nouveau-nés jusqu’à 24 heures après l’accouchement. Davantage d’intérêt devra être porté aux effets secondaires à long terme chez les nouveau-nés au cours des futures études.
Similar content being viewed by others
Labour pain has been considered to be one of the most unbearable pain experiences,1 and many women sustain long-term emotional and psychological effects following childbirth.2 A number of medical procedures have been introduced for pain relief, such as epidural/spinal analgesia, pudendal nerve block, and paracervical blockade. Epidural/spinal analgesia, including epidural analgesia (EA), combined spinal/epidural analgesia, and spinal analgesia are considered to be the most effective procedures.3 With epidural/spinal labour analgesia, local anesthetics (e.g., bupivacaine and ropivacaine) are most commonly used. In addition, the opioids (e.g., meperidine, fentanyl, sufentanil, and remifentanil) are also added to improve the quality of analgesia because of their earlier onset and superior pain relief.4 Nevertheless, the use of opioids for labour analgesia is currently controversial because of the potentially negative effects on neonates.
It is known that opioids can pass easily through the placenta and induce opioids-related effects on newborns. Respiratory depression is probably the most serious side effect induced by opioids. This manifests by minute volume reduction,5 a decrease in oxygen saturation,6 and respiratory acidosis.7 In addition, alterations in neonatal neurobehaviour8 and decreases in the variability of the fetal heart rate9 induced by opioids are also harmful. Among these opioids, meperidine is reported to be the most widely used for labour analgesia10 despite evidence that indicates its limited efficacy and degree of side effects.11 Fentanyl and sufentanil are synthetic opioids which appear to be significantly safer and have less negative impact on neonates.12-14 Nevertheless, some studies15-17 suggest that these synthetic opioids are far from ideal.18-20 Nakamura et al. 15 reported that the use of sufentanil in labour analgesia caused increased pruritus and poor outcomes in newborns when compared with those of the controls. Tian et al. 13 reported that intrathecal sufentanil is safe for both mothers and newborns; however, sometimes it can induce a rise in maternal temperature during labour.
Neonatal outcome is one of the most important concerns relating to labour analgesia. The routine methods to assess the impact of labour analgesia on neonates include a lower Apgar score,13,15,21,22 a lower umbilical artery (UA) or vein (UV) pH value,16,17,23 and a lower Neurological and Adaptive Capacity Score (NACS).24,25 The NACS is a systemic assessment of neonates26 that evaluates five general aspects: adaptive capacity, passive tone, active tone, primary reflexes, and general neurologic status. Systematic reviews have been conducted in an attempt to pool all the data to facilitate analysis of different methods and different anesthetic drugs on the degree of patient satisfaction and the efficacy of labour analgesia.4,27,28 A meta-analysis was conducted to assess the effect of non-axial administration of fentanyl on neonatal outcome;29 however, a quantitative analysis was not conducted, and an evaluation of intrathecal fentanyl was not included.
The objective of this meta-analysis was to include randomized controlled trials (RCTs) addressing epidural/spinal opioids for labour analgesia in order to evaluate their effect on neonatal outcomes. The primary outcome measures were Apgar scores, NACS, and UA/UV pH values. We also attempted to examine patient satisfaction and side effects.
Methods
Search strategy
We searched PubMed (1966 to Oct 2013), EMBASE™ (1966 to Oct 2013), and the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 9, 2013) without language restriction for trials assessing the effects of labour analgesia with opioids on neonatal outcome. The latest search was explicitly updated to Oct 20, 2013. The search strategies included combinations of the following text words: (‘analgesia’ OR ‘anesthesia’) AND (‘obstetrical’ OR ‘pregnancy’ OR ‘labor’) AND (‘neonate’ OR ‘neonatus’ OR ‘newborn’ OR ‘infant’) AND (‘epidural’ OR ‘combined spinal epidural’ OR ‘spinal’ OR ‘intrathecal’) AND (‘opioids’ OR ‘fentanyl’ OR ‘sufentanil’ OR ‘remifentanil’ OR ‘morphine’ OR ‘alfentanil’). The reference lists of the reviews, original reports, case reports, letters to the editor, conference abstracts (Index to Scientific & Technical Proceedings database, 2002 to Oct 2013), and meta-analyses involving the effects of labour analgesia with opioids on the outcomes of neonates were also scanned to avoid missing trials not yet included in the databases.
Study selection
The selection criteria for this meta-analysis were as follows: 1) study design: RCT; 2) participants: healthy women in labour (nulliparous and parous) under epidural/spinal analgesia; 3) interventions: epidural/spinal opioids combined with local anesthetic compared with only local anesthetic administration; 4) outcome variables - at least one of the following was reported: Apgar scores, NACS, UA/UV pH values. Studies were excluded where two arms of the comparison both used opioids in the labour analgesia procedure. Two reviewers (W.K., C.L.) independently evaluated the eligibility of all trials which trended for inclusion in this meta-analysis. Any nonconformity was checked by another author (S.L.Q.).
Data extraction
Two researchers (W.K., C.L.) independently extracted data from the included trials using a predesigned table. Data extraction included publication years, authors of each study, number and age of the participants, technique and drugs of the labour analgesia, visual analogue pain scale (VAPS) of the parturients, and outcomes of the neonates. Graphic digitizing software (Engauge Digitizer version 3.0, http://digitizer.sourceforge.net/) was used to extract data shown in the figures. Any nonconformity was resolved by discussion among all authors.
Qualitative assessment
The quality of all the included studies was appraised using the guidelines recommended by the Cochrane Collaboration.30 The risk of bias was evaluated in six categories: randomization and sequence generation, blinding method, allocation concealment, incomplete outcome data, selective outcome reporting, and other sources of bias. Every category was assessed according to three rulings: low risk, unclear risk, and high risk. Each of the included trials was assigned a classification of quality on the basis of the Cochrane Handbook (Table 8.7.a). The items of randomization and sequence generation, blinding method, and allocation concealment were considered as key domains and the evaluation was as follows: low risk of bias (low risk of bias for all key domains); unclear risk of bias (unclear risk of bias for one or more key domains); and high risk of bias (high risk of bias for one or more key domains). Two authors (W.K., C.L.) independently evaluated the quality of the trials. Any disagreement about the appraisal was resolved by discussion among all authors.
Statistical analysis
The primary outcome measures were Apgar scores, NACS, and UA/UV pH values. Secondary outcomes included VAPS score after labour analgesia and side effects of parturients (motor block, vomiting, pruritus, nausea, sedation, and hypotension). All outcomes were included in the meta-analysis to assess the safety of opioids on neonates and parturients in labour analgesia.
For dichotomous outcomes, we calculated risk differences (RD) and corresponding 95% confidence intervals (CIs) for each trial separately and then pooled the estimates with a fixed-effect model using the Mantel-Haenszel method. We chose a fixed-effect model as it provides a less biased estimate when an outcome is rare, even under conditions of heterogeneity.31,32 For continuous outcomes, we calculated mean differences (MD) and corresponding 95% CIs for each trial separately and then pooled the estimates using a random effects model. We chose a random effects model to account for the clinical and methodological heterogeneity between studies. If the measurement scale was not consistent across studies, we used standardized mean differences (SMD). The mean and standard deviation were calculated when continuous outcomes were summarized as interquartile range, median, and range (Appendix). Statistical heterogeneity was quantified using the I 2 statistic. All reported P values are two-sided. Review Manager 5.2 (Cochrane Collaboration, Oxford, UK) and Stata® 10.0 (Stata Corp, College Station, TX, USA) were used to perform the statistical analysis.
Sensitivity analysis and publication bias
A sensitivity analysis was performed to assess whether inclusion of the high-risk studies could significantly bias the result. The subgroups were conducted according to high and not high risk of bias (including low and unclear risk of bias). To visually assess the potential for publication bias, we constructed a funnel plot for the outcome of Apgar scores at one minute, which involved most of the included trials.
Results
Study selection
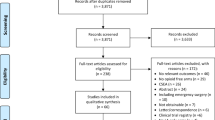
The search of PubMed, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) initially yielded 552 trials. The search strategy was presented in a flow diagram (Fig. 1). Initially, 371 trials were discarded because they were not RCTs or they were duplicates of other studies. After reviewing the abstracts of the remaining 181 trials, 146 were excluded because the trials were either not relevant to our study or both groups used opioids. After reviewing the full texts for a detailed evaluation,14 of the remaining 35 trials were excluded as no endpoint of interest was reported, and in three of these trials, the full text was not found: one in German,33 one in Spanish,34 and one in French.35 Finally, 21 trials13,15-17,21-23,36-49 that met the criteria were included in the meta-analysis. No additional studies, unpublished relevant trials, or abstracts of meetings were obtained.
Study characteristics
The characteristics of these included trials are shown in Table 1. The 21 RCTs comprised 2,859 participants, 1,443 with opioids added to labour analgesia and 1,416 with only local anesthetic. All participants were healthy with American Society of Anesthesiologists physical status I or II and without a history of drug abuse, opioid use, opioid allergy, or other complications. The labour analgesia was usually begun during active first-stage labour. The control group received only a local anesthetic such as bupivacaine, ropivacaine, or lidocaine, and the opioid group received a combination of local anesthesia and opioids. Eight trials reported the total opioid dose.16,17,22,23,40,42-44 Among these, the fentanyl dose was 100-500 µg for EA and up to 1,500 µg for patient-controlled intravenous analgesia; meanwhile, the sufentanil dose for EA was 7.5-30 µg.
Synthesis of results
Apgar scores at one minute
Eighteen trials1,13,15-17,23-46 compared the incidence of Apgar scores < 7 at one minute after delivery; these trials comprised 1,290 women in the opioid group and 1,300 women in the control group (Fig. 2). There was no significant difference between the opioid and control groups (RD 0.0%, 95% CI: −3.0 to 2.0, P = 0.78), which suggested that the use of opioids for labour analgesia did not influence the neonatal outcome of Apgar scores at one minute. With the subgroup analysis, no significant difference was found between the not high (the low and unclear) and high risk of bias groups (P = 0.80). No significant heterogeneity was found in these trials (I 2 = 0%, 95% CI: 0 to 50).
Apgar scores at five minutes
The 18 trials comprised 1,290 women in the opioid group and 1,300 women in the control group.21-23,36-46 These trials compared the incidence of Apgar scores < 7 at five minutes (Fig. 3). No significant difference was found between the two groups (RD −1.0%, 95% CI: −2.0 to 1.0, P = 0.31), which indicated that opioids did not have a significant effect on the neonates. No differences were detected between the subgroups (P = 0.95). No significant heterogeneity was found among these trials (I 2 = 0%, 95% CI: 0 to 50).
Neurological and Adaptive Capacity Scores at two hours
Five trials15-17,23,40 comprising 141women in the opioid group and 135 women in the control group compared NACS at two hours (Fig. 4). These studies found that the status of neonates who were systematically evaluated by NACS at 2 hours showed no difference between the two groups (MD −0.35, 95% CI: −1.70 to 1.01, P = 0.62). In the subgroup analysis, no significant difference was found (P = 0.10); however, it appeared that the NACS values were much lower after the use of opioids in the high risk of bias group. No significant heterogeneity was found among these trials (I 2 = 0%, 95% CI: 0 to 79).
Neurological and Adaptive Capacity Scores at 24 hr
Six trials15-17,22,23,40 comprising 161 women in the opioid group and 155 women in the control group compared NACS at 24 hr (Fig. 5). The overall evaluation showed that opioids did not influence the outcome of NACS at 24 hr (MD −0.45, 95% CI: −1.36 to 0.46, P = 0.33). In the subgroup analysis, the high risk group, but not the low and unclear risk of bias group, showed that opioids could induce lower NACS scores at 24 hr. No significant heterogeneity was found among these trials (I 2 = 3%, 95% CI: 0 to 26).
Umbilical artery or vein pH values
Five trials16,17,23,41,43 reported UA/UV pH values: 271 women in four trials with UA pH values and another 302 women in four trials with UV pH values. The meta-analysis indicated that opioids did not significantly alter the UA pH values (MD −0.02, 95% CI: −0.06 to 0.03, P = 0.48) or the UV pH values (MD −0.03, 95% CI: −0.07 to 0.00, P = 0.08). Obvious heterogeneity was found among these trials, indicating remaining uncertainty about the true treatment effect.
Secondary outcomes
We also performed a meta-analysis on the VAPS scores of women. Nine trials with 478 women were included to assess the efficacy of opioids in labour analgesia. Seven trials used a 0-100 scale, one trial used a 0-10 scale, and another trial used a 0-4 scale of the VAPS scoring system (Table 1); therefore, the SMD was used to assess the effects. The results indicated that additional administration of opioids was superior to the traditional method which only used local anesthetic (SMD −1.95, 95% CI: −3.06 to −0.84, P = 0.0006).
The potential maternal side effects, such as motor block, vomiting, pruritus, nausea, sedation, and hypotension of parturients, were also explored (Table 2). The pooled meta-analysis showed that opioids could induce significant pruritus in parturients; however, the incidence of vomiting, nausea, sedation, and hypotension was not significantly different between the opioid and control groups (Table 3). Motor block was not included in the meta-analysis; however, the individual studies37,38,41,42 showed that opioids did not seem to affect the motor function of the women.
Sensitivity analysis and publication bias
Sensitivity analysis was performed by dividing the studies into high, low, and unclear risk of bias subgroups. Including the trials with a high risk of bias did not bias the results significantly, which meant that the evaluations in our meta-analysis were stable. The funnel plot for the outcome of Apgar scores at one minute was conducted and it did not reveal any asymmetry (Fig. 6).
Discussion
With labour analgesia, in addition to the efficacy of the analgesia and patient satisfaction, safety of the neonates is an important issue. In this review, we investigated the effects of epidural/spinal opioids in labour analgesia on neonatal outcomes. Twenty-one RCTs comprising 2,859 women were included in the meta-analysis.
Primary outcomes
Apgar scores are the most commonly used method to assess newborn status, and they are strong predictors of neonatal mortality.50,51 The scoring system contains five neonatal measurements: activity (muscle tone), pulse, grimace (reflex irritability), appearance (skin colour), and respiration, which are evaluated at one minute and five minutes after birth. Neonates with Apgar scores < 7 might require resuscitative measures. In our meta-analysis, the incidence of Apgar scores < 7 was pooled to clarify whether the opioids given during labour analgesia affected the neonates. The data on Apgar scores at both one minute and five minutes were analyzed, and the results showed no difference between the two groups, which indicated that the opioids do not significantly influence the Apgar scores. The meta-analysis included only one systematic review, which described the relevant data by way of a qualitative summary and concluded that opioids did not affect Apgar scores.29 Our meta-analysis is in agreement with this result; moreover, our review provides a more precise estimate by quantitative analysis of all data.
Neurological and Adaptive Capacity Scores is another scoring system which is more systemic and places more emphasis on neurological status. While Apgar scores may display drug-induced neurological depression, such as mild hypotonia or poor primary reflex responses,52 the NACS was developed to differentiate drug-induced depression as a result of labour trauma, asphyxia, or neurological disease.26 The NACS scoring system contains five aspects, including adaptive capacity, passive tone, active tone, primary reflexes, and general neurologic status. The maximum possible total score is 40, and a score of ≥ 35 indicates a neurologically vigorous newborn. The major time points for measurement of neonatal status are two hours after delivery (short term) and 24 hr after delivery (long term); therefore, we performed the meta-analysis accordingly. The overall evaluation indicated that opioids in labour analgesia did not affect the neurological status of neonates. Two other systematic reviews focused on the NACS. One review53 with seven RCTS compared remifentanil with meperidine for labour analgesia, and the other review27 comprised three RCTs that assessed the effect of EA with fentanyl on neonates. Nevertheless, neither review drew an explicit conclusion regarding the NACS outcome. In our meta-analysis, we enrolled more high-quality RCTs, pooled the data, and obtained a quantitative estimation.
Umbilical artery and vein pH values are critical endpoints to determine fetal acidosis, which is a predicator of perinatal asphyxia.54 The threshold of UA pH ranges from 7.0-7.20, while UV pH ranges from about 7.10-7.20 in most reported RCTs. A systematic review concludes that low umbilical cord pH is substantially associated with neonatal mortality and morbidity and cerebral palsy in childhood.55 In our meta-analysis, no difference was detected between the two groups when the overall effects were assessed. Meanwhile, the mean values of all UA/UV pH values were above 7.23, which indicated that both the traditional and additional opioids methods did not induce significant side effects on neonates. In a meta-analysis comparing an intermittent epidural bolus with continuous epidural infusions for labour analgesia, no difference was detected in UA/UV pH values.4 In another study, no difference was found in UA/UV pH values when remifentanil was compared with meperidine in labour analgesia.53
Secondary outcomes
Patient satisfaction and side effects, important factors in the assessment of the quality of labour analgesia, have been evaluated in other systematic reviews and meta-analyses. George et al. reported that administering an intermittent epidural bolus was superior to a continuous epidural infusion, as measured by the VAPS score.4 Leong et al. reported that the VAPS score was higher in the remifentanil group than in the meperidine group at one hour after injection.53 Schnabel et al. indicated that remifentanil was more effective than pethidine, and that remifentanil continuous EA is superior to patient-controlled analgesia.56 In our meta-analysis, we focused on evaluating the efficacy of epidural/spinal opioids with labour analgesia and found that the addition of opioids to a local anesthetic can significantly improve the VAPS score.
The common maternal side effects induced by opioids include motor block, vomiting, pruritus, nausea, sedation, and hypotension. In our study, we displayed all of the data in the individual RCTs and performed a meta-analysis. The results indicated that opioids did not induce any side effects other than significant pruritus. In addition, the results were in agreement with other studies that focused on the side effects of opioids.57-59 After making a trade-off between neonate and maternal outcomes, in our view, labour analgesia with opioids is worth considering, especially an epidural/spinal method.
Limitations and future directions of the study
In our meta-analysis, the methods and duration of opioid administration varied amongst the individual studies, which was probably a source of the heterogeneity. In addition, the RCTs which met the inclusion criteria concerned only fentanyl and sufentanil. Consequently, the effects of other opioids, such as remifentanil and morphine, were not included in our review. Furthermore, all outcomes were measured within 24 hr after delivery, therefore the long-term effects of opioids on neonates could not be evaluated. As previously mentioned, more well-designed RCTs should focus on these factors to add additional clarification.
In conclusion, the results of this meta-analysis showed that the commonly administered doses of fentanyl and sufentanil for labour analgesia are safe up to 24 hr after delivery. Future studies should focus on the long-term neonatal side effects.
References
Melzack R. The myth of painless childbirth (the John J. Bonica lecture). Pain 1984; 19: 321-37.
Lavand’homme P. Chronic pain after vaginal and cesarean delivery: a reality questioning our daily practice of obstetric anesthesia. Int J Obstet Anesth 2010; 19: 1-2.
Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev 2011; 12: CD000331.
George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg 2013; 116: 133-44.
Kane KM, Percival N, Please NW, Roberts H, Snow P. Effects of some analgesic drugs used in childbirth; with special reference to variation in respiratory minute volume of the newborn. Lancet 1957; 272: 128-32.
Hamza J, Benlabed M, Orhant E, et al. Neonatal pattern of breathing during active and quiet sleep after maternal administration of meperidine. Pediatr Res 1992; 32: 412-6.
Koch G, Wendel H. The effect of pethidine on the postnatal adjustment of respiration and acid base balance. Acta Obstet Gynecol Scand 1968; 47: 27-37.
Hodgkinson R, Bhatt M, Wang CN. Double-blind comparison of the neurobehaviour of neonates following the administration of different doses of meperidine to the mother. Can Anaesth Soc J 1978; 25: 405-11.
Kariniemi V, Ammala P. Effects of intramuscular pethidine on fetal heart rate variability during labour. Br J Obstet Gynaecol 1981; 88: 718-20.
O’Sullivan G. Analgesia and anaesthesia in labour. Curr Obstet Gynaecol 2005; 15: 9-17.
Evron S, Ezri T. Options for systemic labor analgesia. Curr Opin Anaesthesiol 2007; 20: 181-5.
Rayburn WF, Smith CV, Parriott JE, Woods RE. Randomized comparison of meperidine and fentanyl during labor. Obstet Gynecol 1989; 74: 604-6.
Tian F, Wang K, Hu J, et al. Continuous spinal anesthesia with sufentanil in labor analgesia can induce maternal febrile responses in puerperas. Int J Clin Exp Med 2013; 6: 334-41.
Akkamahadevi P, Srinivas H, Siddesh A, Kadli N. Comparision of efficacy of sufentanil and fentanyl with low-concentration bupivacaine for combined spinal epidural labour analgesia. Indian J Anaesth 2012; 56: 365-9.
Nakamura G, Ganem EM, Rugolo LM, Castiglia YM. Effects on mother and fetus of epidural and combined spinal-epidural techniques for labor analgesia. Rev Assoc Med Bras 2009; 55: 405-9.
Loftus JR, Hill H, Cohen SE. Placental transfer and neonatal effects of epidural sufentanil and fentanyl administered with bupivacaine during labor. Anesthesiology 1995; 83: 300-8.
Porter J, Bonello E, Reynolds F. Effect of epidural fentanyl on neonatal respiration. Anesthesiology 1998; 89: 79-85.
Douma MR, Middeldorp JM, Verwey RA, Dahan A, Stienstra R. A randomised comparison of intravenous remifentanil patient-controlled analgesia with epidural ropivacaine/sufentanil during labour. Int J Obstet Anesth 2011; 20: 118-23.
Malvasi A, Tinelli A, Brizzi A, et al. Intrapartum sonography for occiput posterior detection in early low dose combined spinal epidural analgesia by sufentanil and ropivacaine. Eur Rev Med Pharmacol Sci 2010; 14: 799-806.
Kalra S, Saraswat N, Agnihotri GS. Comparison of efficacy of bupivacaine and fentanyl with bupivacaine and sufentanil for epidural labor analgesia. Saudi J Anaesth 2010; 4: 178-81.
Salim R, Nachum Z, Moscovici R, Lavee M, Shalev E. Continuous compared with intermittent epidural infusion on progress of labor and patient satisfaction. Obstet Gynecol 2005; 106: 301-6.
Beilin Y, Bodian CA, Weiser J, et al. Effect of labor epidural analgesia with and without fentanyl on infant breast-feeding: a prospective, randomized, double-blind study. Anesthesiology 2005; 103: 1211-7.
Nikkola EM, Jahnukainen TJ, Ekblad UU, Kero PO, Salonen MA. Neonatal monitoring after maternal fentanyl analgesia in labor. J Clin Monit Comput 2000; 16: 597-608.
Gambling D, Berkowitz J, Farrell TR, Pue A, Shay D. A randomized controlled comparison of epidural analgesia and combined spinal-epidural analgesia in a private practice setting: pain scores during first and second stages of labor and at delivery. Anesth Analg 2013; 116: 636-43.
Lilker S, Rofaeel A, Balki M, Carvalho JC. Comparison of fentanyl and sufentanil as adjuncts to bupivacaine for labor epidural analgesia. J Clin Anesth 2009; 21: 108-12.
Amiel-Tison C, Barrier G, Shnider SM, Levinson G, Hughes SC, Stefani SJ. A new neurologic and adaptive capacity scoring system for evaluating obstetric medications in full-term newborns. Anesthesiology 1982; 56: 340-50.
Jones L, Othman M, Dowswell T, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database Syst Rev 2012; 3: CD009234.
Bauer ME, Kountanis JA, Tsen LC, Greenfield ML, Mhyre JM. Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: a systematic review and meta-analysis of observational trials. Int J Obstet Anesth 2012; 21: 294-309.
Fleet J, Jones M, Belan I. Non-axial administration of fentanyl in childbirth: a review of the efficacy and safety of fentanyl for mother and neonate. Midwifery 2011; 27: e106-13.
Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration: Wiley Online Library; 2008 .
Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 2007; 7: 5.
Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985; 41: 55-68.
Vettermann J, Thomas H, Lischke V, Asskali F. Repeated addition of fentanyl to bupivacaine peridural analgesia in labor. Clinical action and fentanyl plasma level (German). Anaesthesist 1996; 45: 428-36.
Melchor JC, Ruiz A, Santos C, et al. Comparative study of peridural anesthesia with bupivacaine alone and combined with fentanyl during vaginal delivery (Spanish). Rev Esp Anestesiol Reanim 1991; 38: 80-2.
Desprats R, Mandry J, Grandjean H, Amar B, Pontonnier G, Lareng L. Peridural analgesia during labor: comparative study of a fentanyl-marcaine combination and marcaine alone (French). J Gynecol Obstet Biol Reprod (Paris) 1983; 12: 901-5.
Pace MC, Aurilio C, Bulletti C, Iannotti M, Passavanti MB, Palagiano A. Subarachnoid analgesia in advanced labor: a comparison of subarachnoid analgesia and pudendal block in advanced labor: analgesic quality and obstetric outcome. Ann N Y Acad Sci 2004; 1034: 356-63.
Lee BB, Ngan Kee WD, Lau WM, Wong AS. Epidural infusions for labor analgesia: a comparison of 0.2% ropivacaine, 0.1% ropivacaine, and 0.1% ropivacaine with fentanyl. Reg Anesth Pain Med 2002; 27: 31-6.
Ruban P, Sia AT, Chong JL. The effect of adding fentanyl to ropivacaine 0.125% on patient-controlled epidural analgesia during labour. Anaesth Intensive Care 2000; 28: 517-21.
Olofsson C, Ekblom A, Ekman-Ordeberg G, Irestedt L. Obstetric outcome following epidural analgesia with bupivacaine-adrenaline 0.25% or bupivacaine 0.125% with sufentanil—a prospective randomized controlled study in 1000 parturients. Acta Anaesthesiol Scand 1998; 42: 284-92.
Claes B, Soetens M, Van Zundert A, Datta S. Clonidine added to bupivacaine-epinephrine-sufentanil improves epidural analgesia during childbirth. Reg Anesth Pain Med 1998; 23: 540-7.
Steinberg RB, Dunn SM, Dixon DE, Rehm KL, Pastides H, Xu X. Comparison of sufentanil, bupivacaine, and their combination for epidural analgesia in obstetrics. Reg Anesth 1992; 17: 131-8.
Vertommen JD, Vandermeulen E, Van Aken H, et al. The effects of the addition of sufentanil to 0.125% bupivacaine on the quality of analgesia during labor and on the incidence of instrumental deliveries. Anesthesiology 1991; 74: 809-14.
Murphy JD, Henderson K, Bowden MI, Lewis M, Cooper GM. Bupivacaine versus bupivacaine plus fentanyl for epidural analgesia: effect on maternal satisfaction. BMJ 1991; 302: 564-7.
Viscomi CM, Hood DD, Melone PJ, Eisenach JC. Fetal heart rate variability after epidural fentanyl during labor. Anesth Analg 1990; 71: 679-83.
Jones G, Paul DL, Elton RA, McClure JH. Comparison of bupivacaine and bupivacaine with fentanyl in continuous extradural analgesia during labour. Br J Anaesth 1989; 63: 254-9.
Van Steenberge A, Debroux HC, Noorduin H. Extradural bupivacaine with sufentanil for vaginal delivery. A double-blind trial. Br J Anaesth 1987; 59: 1518-22.
Marcos Vidal JM, Gutierrez Fernandez A, Ceron Pena L, Baticon Escudero PM, Gutierrez Fernandez J, Mourad MM. Comparison of intrathecal fentanyl and bupivacaine in combined spinal-epidural obstetric analgesia (Spanish). Rev Esp Anestesiol Reanim 2008; 55: 341-7.
Jorrot JC, Lirzin JD, Dailland P, Jacquinot P, Conseiller C. A combination of sufentanil and 0.25% bupivacaine administered epidurally for obstetrical analgesia. Comparison with fentanyl and placebo (French). Ann Fr Anesth Reanim 1989; 8: 321-5.
Milon D, Lavenac G, Noury D, et al. Epidural anesthesia during labor: comparison of 3 combinations of fentanyl-bupivacaine and bupivacaine alone (French). Ann Fr Anesth Reanim 1986; 5: 18-23.
Chong DS, Karlberg J. Refining the Apgar score cut-off point for newborns at risk. Acta Paediatr 2004; 93: 53-9.
Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953; 32: 260-7.
Scanlon JW, Brown WU Jr, Weiss JB, Alper MH. Neurobehavioral responses of newborn infants after maternal epidural anesthesia. Anesthesiology 1974; 40: 121-8.
Leong WL, Sng BL, Sia AT. A comparison between remifentanil and meperidine for labor analgesia: a systematic review. Anesth Analg 2011; 113: 818-25.
Fahey J, King TL. Intrauterine asphyxia: clinical implications for providers of intrapartum care. J Midwifery Womens Health 2005; 50: 498-506.
Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ 2010; 340: c1471.
Schnabel A, Hahn N, Broscheit J, et al. Remifentanil for labour analgesia: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol 2012; 29: 177-85.
Landau R, Liu SK, Blouin JL, Carvalho B. The effect of OPRM1 and COMT genotypes on the analgesic response to intravenous fentanyl labor analgesia. Anesth Analg 2013; 116: 386-91.
Jaskot B, Czeszynska Maria B, Konefal H, Pastuszka J. Method of analgesia for labor in relation to newborn condition, cord blood cortisol and interleukin-6 levels (Polish). Ginekol Pol 2011; 82: 767-74.
Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg 2011; 113: 826-31.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2014; 61: this issue.
Author contributions
Kai Wang, Liang Cao, Jie Song, and Dun-Yi Qi helped design the study. Kai Wang, Liang Cao, and Dun-Yi Qi helped conduct the study. Kai Wang, Liang Cao, Qian Deng, and Tianyu Gu helped analyze the data. Kai Wang, Liang Cao, and Tianyu Gu helped write the manuscript. Kai Wang and Liang Cao contributed equally to this work. Li-Qiang Sun helped with the literature collection.
Appendix
Appendix
The formulas used to calculate standard deviation (SD) in this meta-analysis are listed below:
(1) The formula can be used to estimate the SD from the standard error of the mean (SEM) and the sample size (n).
(2) The formula can be used to estimate the mean using the median (m) and the low and high end of the range (a and b, respectively).60
The SD (S) can be estimated by
(3) The formula can be used to estimate pooled SD (S) from separate SD1 (S1), n1 and SD2 (S2), n2 …
Rights and permissions
About this article
Cite this article
Wang, K., Cao, L., Deng, Q. et al. The effects of epidural/spinal opioids in labour analgesia on neonatal outcomes: a meta-analysis of randomized controlled trials. Can J Anesth/J Can Anesth 61, 695–709 (2014). https://doi.org/10.1007/s12630-014-0185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0185-y