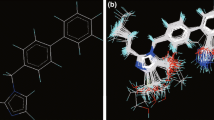
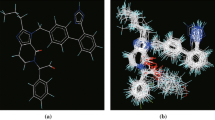
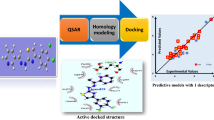
Abstract
A series of oxadiazole-substituted α-isopropoxy phenylpropanoic acids with dual activators of PPARα and PPARγ derivatives were subjected to two dimensional and k-nearest neighbour Molecular field analysis. The statistically significant best 2D-QSAR (PPARα) model having good predictive ability with statistical values of r2 = 0.8725, q2 = 0.7957 and pred_r2 = 0.8136, was developed by GA-PLS with the descriptors like SsClcount, SddsN (nitro) count and SsOHcount contribute significantly to the biological activity. The best 3D-QSAR studies (PPARα) were performed using the genetic algorithm selection k-nearest neighbor molecular field analysis approach; a leave-one-out cross-validated correlation coefficient q2=0.7188 and predicate activity pred_r2 = 0.7508 were obtained. The influences of steric and electrostatic field effects generated by the contribution plots are discussed. The best pharmacophore model includes three features viz. hydrogen bond donor, hydrogen bond acceptor, and aromatic features were developed. The information rendered by 2D, 3D QSAR models may lead to a better understanding of structural requirements of substituted α-isopropoxy phenylpropanoic derivatives and also aid in designing novel potent PPARα and PPARγ for antihyperglycemic molecules.
Similar content being viewed by others
References
Amos AF, McCarty DJ, Zimmet P (1997) The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 14(5): S1–85
Ajmani S, Jadhav K, Kulkarni SA (2006) Three-Dimensional QSAR using the k-Nearest Neighbor method and its interpretation. J Chem Inf Model 46: 24–31.
Baumann K (2000) an alignment-independent versatile structure descriptor for QSAR and QSPR based on the distribution of molecular features. J Chem Inf Comput Sci 42: 26–35.
Basu-Modak S, Braissant O, Escher P, Desvergne B, Honegger P, Wahli W (1999) Peroxisome Proliferator- Activated Receptor β Regulates Acyl-CoA Synthetase 2 in Reaggregated Rat Brain Cell Cultures. J Biol Chem 274: 35881-35888.
Berger J, Bailey P, Biswas C, Cullinan C A, Doebber TW, Hayes N S, Saperstein R, Smith R G, Leibowitz MD (1996) Thiozolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-γ: binding and activation correlates with antidiabetic actions in db/db mice. Endocrinology 137: 4189–4195.
Brun RP, Kim JB, Hu E, Spiegelman BM (1997) Peroxisome proliferator-activated receptor gamma and the control of adipogenesis. Curr Opin Lipidol 8(4): 212–8.
Buckle DR, Cantello BCC, Cawthorne M A, Coyle P J, Dean D K, Faller A, Haigh D, Hindley R M, Jefcott L J, Lister C A, Pinto I L, Rami H K, Smith D G, Smith S A (1996) Nonthiazolidinedione Antihyperglycemic Agents. 1: α-Heteroatom Substituted β-Phenylpropanoic Acids. Bioorg Med Chem Lett 6: 2121–2126.
Bhatia MS, Pakhare KD, Choudhari PB, Jadhav S D, Dhavale, RP, Bhatia NM (2012) Pharmacophore modeling and 3D QSAR studies of aryl amine derivatives as potential lumazine synthase inhibitors. Arab J Chem https://doi.org/10.1016/j.arabjc.2012.05.008.
Cramer RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA) 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110: 5959–5967.
Clark M, Cramer RD III, Van ON (1989) Validation of the general purpose tripos 5.2 force field. J Comput Chem 10: 982–1012.
Choudhari P, Bhatia M (2012) 3D QSAR, pharmacophore indentification studies on series of 1- (2-ethoxyethyl)-1Hpyrazolo [4, 3-d] pyrimidines as phosphodiesterase V inhibitors. J Saudi Chem Soc https://doi.org/10.1016/j.jscs.2012.02.008.
Collins J L, Blanchard SG, Boswell EG, Charifson PS, Cobb JE, Henke B R, Hull-Ryde E A, Kazmierski W W, Lake DH, Leesnitzer L M, Lehmann J, Lenhard J M, Orband-Miller L A, Gray-Nunez Y, Parks D J, Plunket KD, Tong, W (1998) N-(2-Benzoylphenyl)-Ltyrosine PPAR-Cc Agonists. 2. Structure-Activity Relationship and Optimization of the Phenyl Alkyl Ether Moiety. J Med Chem 41: 5037–5054.
Dow R L, Bechle BM, Chou T T, Clark DA, Hulin B, Stevenson RW (1991) Benzyloxazolidine-2, 4-dines as Potent Hypoglycemic Agents. J Med Chem 34: 1538–1554.
Diamant M, Heine RJ (2003) Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs 63(13): 1373–405.
Ferreira MMC (2002) Multivariate QSARh. J. Braz. Chem. Soc., 13, 742–753.
Gross B, Staels B (2007) PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endo crinol Meta 21(4): 687–710.
Golbraikh A, Tropsha A (2002) Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection. J Comput Aided Mol Des 16: 357–369.
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity-a rapid access to atomic charges. Tetrahedron 36: 3219–3228.
Hasegawa K, Kimura T, Funatsu K (1999) GA Strategy for Variable Selection in QSAR Studies: Enhancement of Comparative Molecular Binding Energy Analysis by GA-Based PLS Method. Quant Struct Act Relat 18: 262–272.
Henke B R (2004) Proxisome Proliferators-Activated Receptor α/γ Dual Agonists for the Treatment of Type 2 Diabetes. J Med Chem 47, 4118–4127.
Holland J H (1992) Genetic algorithms. Sci Am 267: 66–72.
Halgren TA (1996) Merck molecular force field. III. Molecular geometries and vibrational frequencies for MMFF94. J Comput Chem 17: 553–586.
Jönsson B (2002) Revealing the cost of Type II diabetes in Europe. Diabetologia 45, S5–12.
Joy SV, Rodgers PT, Scates AC (2005) Incretinmimetics as emerging treatments for type 2 diabetes. Ann Pharmacother 39, 110–118.
Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE (2005) (2R)-4-oxo-4-[3-(trifluoromethyl)-5, 6-dihydro[1, 2, 4]triazolo[4, 3- a]pyrazin-7(8H)-yl]-1-(2, 4, 5 trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48: 141–151.
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405, 421–424.
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes. Care 21: 1414–1431.
Liu KG, Smith JS, Ayscue AH, Henke BR, Lambert MH, Leesnitzer LM, Plunket KD, Willson TM, Sternbach DD (2001) Identification of a series of oxadiazolesubstituted alpha-isopropoxyphenylpropanoic acids with activity on PPARα, PPARγ, and PPARδ. Bioorg Med Chem Lett 11: 2385–2388.
Lemberger T, Desvergne B, Wahli W (1996) Peroxisome Proliferator-Activated Receptors: A Nuclear Receptor Signaling Pathway in Lipid Physiology. Annu Rev Cell Dev Biol 12: 335–363.
Lohray B B, Bhushan V, Bajji A C, Kalchar S, Poondra R R, Padakanti S, Chakrabarti R, Vikramadityan R K, Mishra P, Juluri S, Mamidi N V S R, Rajagopalan R (1999) (-)3- [4-[2-(Phenoxazin-10-yl) ethoxy]phenyl]-2-ethoxypropanoic Acid [(-)DRF 2725]: A Dual PPAR Agonist with Potent Antihyperglycemic and Lipid Modulating Activity. J Med Chem 42: 2569–2581.
Leach AR, Gillet VJ (2003) An Introduction to Chemoinformatics. Kluwer, Boston, pp 79–81.
Lehmann J M, Moore LB, Smith-Oliver T A, Wilkison WO, Willson TM, Kliewer S A (1995) An Antidiabetic Thiazolidinedione is a High Affinity Ligand for Peroxisome Proliferator-Activated Receptor γ (PPAR γ). J Biol Chem 270: 12953–12956.
Mangelsdorf DJ, Evans RM (1995) The RXR heterodimers and orphan receptors. Cell 83: 841–50.
Murakami K, Tobe K, Ide T, Mochizuki T, Ohashi M, Akanuma Y, Yazaki Y, Kadowaki T (1998) A novel insulin sensitizer acts as a colig and for peroxisome proliferator-activated receptor-alpha (PPARalpha) and PPAR-gamma: effect of PPAR-alpha activation on abnormal lipid metabolism in liver of Zucker fatty rats. Diabetes 47: 1841–7.
Momose Y, Maekawa T, Yamano T, Kawada M, Odaka H, Ikeda H, Sohda T (2002) Novel 5-Substituted 2, 4-Thiazolidinedione and 2, 4-Oxazolidinedione Derivatives as Insulin Sensitizers with Antidiabetic Activities. J Med Chem 45: 1518–1534.
Nissen S E, Wolski K, Topol E J (2005) Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. J Am Med Assoc 294: 2581–2586.
Oberfield J L, Collins J L, Holmes C P, Goreham D M, Cooper J P, Cobb J E, Lenhard J M, Hull-Ryde EA, Mohr C P, Blanchard S G, Parks D J, Moore L B, Lehmann JM, Plunket K, Miller A B, Milburn MV, Kliewer S AM., Kliewer WT (1999) A peroxisome proliferatoractivated receptor γ ligand inhibits adipocyte differentiation. Proc Nat Aca Sc 96: 6102–6106.
Oliver W R Jr, Shenk J L, Snaith M R, Russell C S, Plunket K D, Bodkin N L, Lewis M C, Winegar DA, Sznaidman ML, Lambert M H, Xu H E, Sternbach D D, Kliewer S A, Hansen B C, Willson T M (2001) A Selective Peroxisome Proliferator- Activated Receptor δ Agonist Promotes Reverse Cholesterol Transport. Proc Natl Acad Sci USA 98: 5306–5311.
Park BH, Vogelstein B, Kinzler K W (2001) Genetic Disruption of PPAR Decreases the Tumorigenicity of Human Colon Cancer Cells. Proc Natl Acad Sci USA 98: 2598–2603.
Ram VJ (2003) Therapeutic role of peroxisome proliferator-activated receptors in obesity, diabetes and inflammation. Prog Drug Res 60: 93–132.
Sauerberg P, Pettersson I, Jeppesen L, Bury P S, Mogensen J P, Wassermann K, Brand C L, Sturis J, Woldike H F, Fleckner J, Andersen A S, Mortensen S B, Svensson L A, Rasmussen H B, Lehmann S V, Polivka Z, Sindelar K, Panajotova V, Ynddal L, Wulff E M (2002) Novel tricyclic-alpha alkyloxyphenylpropionic acids: dual PPARα/γ agonists with hypolipidemic and antidiabetic activity. J Med Chem 45: 789–804.
Sotriffer CA, Winger RH, Liedl KR, Rode BM, Varga JM., 1996. Comparative docking studies on ligand binding to the multispecific antibodies IgE-La2 and IgE-Lb4. J Comput Aided Mol Des 10(4): 305–20.
Sohda T K M, Imamiya E, Sugiyama Y, Fujita T, Kawamatsu Y (1982) Studies on Antidiabetic Agents. II. Syhthesis of 5-[4-(1- Methylcyclohexylmethoxybenzyl]thiazolidine-2, 4-dione (ADD-3878) and Its Derivatives. Chem Pharm Bull 3580–3600.
Shinkai H, Onogi S, Tanaka M, Shibata T, Iwao M, Wakitani K, Uchida I (1998) Isoxazolidine-3, 5-dione and Noncyclic1, 3-Dicarbonyl Compounds as Hypoglycemic Agents. J Med Chem 41: 1927–1933.
Spiegelman BM (1998) PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 47: 507–514.
Smith A, Fogelfeld L, Bakris G (2000). New therapies in diabetes - thiazolidinediones. Emerg Drugs 5: 441–456.
Shearer B G, Billin A N (2007) The next generation of PPAR drugs: do we have the tools to find them? Biochim Biophys Acta 1771: 1082–93.
Staels B, Fruchart J C (2005) Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 54: 2460–70.
VLife MDS 3.5 (2008) Molecular design suite. Vlife SciencesTechnologies Pvt. Ltd., Pune.
World Health Organization, Fact Sheet No. 138, April 2002.
Willson T M, Cobb J E, Cowan D J, Wiethe R W, Corea I D, Prakash SR Beck K D, Moore L B, Kliver S A, Lehman J M (1996) The structure-activity relationship between peroxisome proliferator-activatied receptor γ agonism and the antihyperglycemic activity of thiozolidinediones. J Med Chem 39: 665–668.
Wagman AS, Nuss JM. 2001. Current therapies and emerging targets for the treatment of diabetes. Curr. Pharm. Des. 7: 417–50.
Wang Y X, Zhang C L, Yu R T, Cho H K, Nelson M C, Bayuga-Ocampo C R, Ham J, Kang H, Evans RM (2004) Regulation of Muscle Fiber Type and Running Endurance by PPARδ. PLoS Biol 2: E294.
Zheng W, Tropsha A (2000) Novel variable selection quantitative structure-property relationship approach based on the k-nearest neighbor principle. J Chem Inf Comput Sci 40: 185–194.
Acknowledgment
The author thanks Vlife Science Technologies Pvt. Ltd for providing the trial version software for the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, M.C. Prospective QSAR-based Prediction Models with Pharmacophore Studies of Oxadiazole-substituted α-isopropoxy Phenylpropanoic Acids on with Dual Activators of PPARα and PPARγ. Interdiscip Sci Comput Life Sci 7, 335–346 (2015). https://doi.org/10.1007/s12539-015-0009-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-015-0009-y