Abstract
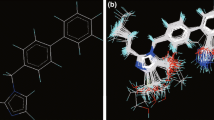
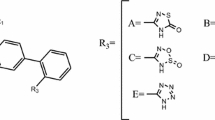
QSAR studies were performed for correlating the chemical composition of 3-(4-biphenylmethyl) 4, 5-dihydro-4-oxo-3H-imidazo [4, 5-c] pyridines bearing aryl acetic acid esters and acetamides as angiotensin II AT\(_{1}\) receptor antagonist. Four different quantitative structure–property relationship (QSAR) methods namely two-dimensional (2D-QSAR), group-based QSAR, k-nearest neighbor and Pharmacophore Modeling were employed to obtain statistically significant models. The statistically significant best 2D-QSAR model having correlation coefficient \(r^{2} = 0.8940\) and cross-validated squared correlation coefficient \(q^{2}=0.7648\) with external predictive ability of pred_\(r^{2}=0.8177\), pred_\(r^{2}\)se = 0.4119 and best group-based QSAR model having \(r^{2}=0.7392\) and \(q^{2}=0.6710\) with pred_\(r^{2}=0.7503\) was developed by SA–principal component regression. The most predictive k-nearest neighbor model derived from the superposition of conformations has good cross-validated \(q^{2}=0.7637\) and satisfied predictive ability \(r^{2}\)_pred = 0.7143. Continuing with compounds of substituted 4, 5-dihydro-4-oxo-3H-imidazo [4, 5-c] pyridine derivatives chemical feature-based pharmacophore models with lowest RMSD value (0.3292 Å) consists of two Hac (Hydrogen bond acceptor), negative ionizable, and two AroC (Aromatic) features are important for the activity. The study suggested that substitution of group at R, R 1, R 2 and Ar, and position on 4, 5-dihydro-4-oxo-3H-imidazo [4, 5-c] pyridine ring with more electronegative nature and low bulkiness are favorable for the antihypertensive activity. These theoretical results may provide a useful reference for understanding the action mechanism and designing potential angiotensin II (AT1) receptor antagonist.





Similar content being viewed by others
References
Welsh L, Ferro A (2004) Drug treatment of essential hypertension: the case for initial combination therapy. Int J Clin Pract 58:956–963
Ferrario CM (1990) The renin-angiotensin system: importance in physiology and pathology. J Cardiovascular Pharmacol 15(3):51–55
Vallotton MB (1987) The renin-angiotensin system. Trends Pharmacol Sci 8:69
Gaddum JH, Hameed KA, Hathway DE, Stephens FF (1955) Quantitative studies of antagonists for 5-hydroxytryptamine. Q J Exp Physiol Cogn Med Sci 40:49–74
Lew MJ, Ziogas J, Christopoulos A (2000) Dynamic mechanisms of non-classical antagonism by competitive AT(1) receptor antagonists. Trends Pharmacol Sci 21:376–381
Morsing P (1999) Candesartan: a new-generation angiotensin II AT1 receptor blocker: pharmacology, antihypertensive efficacy, renal function, and renoprotection. J Am Soc Nephrol Suppl 11:248–254
Timmermans PB, Wong PC, Chiu AT, Herblin WF (1991) Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci 12:55–62
Birkenhäger WH, de Leeuw PW (1999) Non-peptide angiotensin type 1 receptor antagonists in the treatment of hypertension. J Hypertens 17:873–881
Nickenig G, Harrison DG (2002) The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation 105:393–396
Gasparo MD, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharm Rev 52:415–472
Furakawa Y (1982) Hypotensive imidazole derivatives and hypotensive imidazole-5-acetic acid derivates. (U.S. Patent 4340 598 and U.S. Patent 4355 040)
Carini DJ, Duncia JV, Aldrich PE, Chiu AT, Johnson AL, Pierce ME, Price WA, Wells JBGJ, Wexler RR, Wong PC, Yoo S, Timmermans PBMWM (1991) Nonpeptide angiotensin II receptor antagonists: the discovery of series of N- (biphenyl methyl) imidazoles as potent, orally active antihypertensive. J Med Chem 34:2525–2547
Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD (1993) Angiotensin II receptors and angiotensin II receptor antagonists. Pharma Rev 45:205–251
Mancia G, Rosei EA, Cifkova R, DeBacker G, Erdine S, Fagard R, Farsang C, Heagerty AM, Kawecka-Jaszcs K, Kiowski W, Kjeldsen S, Luscher T, McInnes G, Mallion JM, Brien EO, Poulter NR, Priori SG, Rahn KH, Rodicio JL, Ruilope LM, Safar M, Staessen JA, van Zwieten P, Waeber B, Williams B, Zanchetti A, Zannad F (2003) European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 21:1011–1053
Aslam S, Santha T, Leone A, Wilcox C (2006) Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int 70:2109–2115
Cheng QN, Law PK, de Gasparo M, Leung PSJ (2008) Combination of the dipeptidyl peptidase IV inhibitor LAF237 [(S)-1-[(3-Hydroxy-1-adamantyl)ammo]acetyl-2-cyano-pyrrolidine] with the angiotensin II type 1 receptor antagonist valsartan [N- (1-Oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)-[1, 1′-biphenyl]-4-yl]methyl]-L-valine] enhances pancreatic islet morphology and function in a mouse model of type 2 diabetes. Pharmacol Exp Ther 327:683–691
Katayama K, Nomura S, Ishikawa H, Murata T, Koyabu S, Nakano T (2006) Comparison between valsartan and valsartan plus cilnidipine in type II diabetics with normo- and micro albuminuria. Kidney Int 70:151–156
Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM, Investigators VTN (2003) Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 349:1893–1906
Yang GF, Huang X (2006) Development of quantitative structure-activity relationships and its application in rational drug design. Curr Pharm Design 12:4601–4611
Martin YC (1991) Overview of concepts and methods in computer assisted rational drug design. Methods Enzymol 203:587–613
Tropsha A (2003) History of quantitative structure activity relationships. In: Abraham DJ (ed) Burger’s medicinal chemistry and drug discovery. Wiley Interscience, Hoboken
Ajmani S, Jadhav K, Kulkarni SA (2006) Three-dimensional QSAR using the k-nearest neighbor method and its interpretation. J Chem Inf Model 46:24–31
Vlife MDS software (2008) Version 3.5, supplied by Vlifescience technologies Pvt. Ltd, Pune
Mederski WWKR, Dorseh D, M. Osswald NB, Lues I, Minck KO, Schelling P, Ladstetter BJ (1995) 4, 5-Dihydro-4-oxo-3H-imidazo[4, 5-c]pyridines: potent arylacetic acid-derived AT 1 antagonists with improved affinity for the AT2receptor. Bioorg Med Chem Lett 5(22):2665–2670
Halgren TA (1996) Merck molecular force field. III. Molecular geometries and vibrational frequencies for MMFF94. J Comput Chem 17:553–586
Baumann K (2002) an alignment-independent versatile structure descriptor for QSAR and QSPR based on the distribution of molecular features. J Chem Inf Comput Sci 42:26–35
Golbraikh A, Tropsha A (2002) Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection. J Comput Aided Mol Des 16:357–369
Ajmani S, Jadhav K, Kulkarni SA (2009) Group-based QSAR (GQSAR): mitigating interpretation challenges in QSAR. QSAR Comb Sci 28:36–41
Ajmani S, Agrawal A, Kulkarni SA (2010) A comprehensive structure-activity analysis of protein kinase B-alpha (Akt1) inhibitors. J Mol Graph Model 28:683–694
Clark M, Cramer RD III, Van ON (1989) Validation of the general purpose Tripose 5.2 force field. J Comput Chem 10:982–1012
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity-a rapid access to atomic charges. Tetrahedron 36:3219–3228
Ghosh P, Bagchi MC (2009) QSAR modeling for quinoxaline derivatives using genetic algorithm and simulated annealing based feature selection. Curr Med Chem 16:4032–4048
Zheng W, Tropsha A (2000) Novel variable selection quantitative structure-property relationship approach based on the k-nearest neighbor principle. J Chem Inf Comput Sci 40:185–194
Kirkpatrick S, Gelatt CDJ, Vecchi MP (1983) Optimization by simulated annealing. Science 220:671–680
Cramer RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA) 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110:5959–5967
Acknowledgments
The authors are thankful to Vlife Science Technologies Pvt. Ltd. (Pune India) for providing the trial version software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, M.C. QSAR studies on 3-(4-biphenylmethyl) 4, 5-dihydro-4-oxo-3H-imidazo [4, 5-c] Pyridine derivatives as angiotensin II (AT1) receptor antagonist. Interdiscip Sci Comput Life Sci 7, 113–128 (2015). https://doi.org/10.1007/s12539-015-0005-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-015-0005-2