Abstract
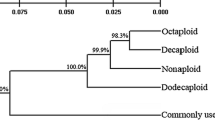
Saccharum spontaneum L. is an important sugarcane germplasm resource with excellent properties of cold, drought, barren, insect and disease resistance. To improve the utilization efficiency of S. spontaneum in sugarcane breeding and variety improvement, the genetic diversity and phylogenetic relationship of 70 S. spontaneum species with different ploidy levels (2n = 40, 48, 54, 64, 80, 88, 92 and 96) from Tibet of China, Nepal, and Myanmar were analyzed using sequence-related amplified polymorphism markers and capillary electrophoresis. The results showed that the average number of polymorphic loci and the proportion of polymorphic loci in polymerase chain reaction amplification results using 16 pairs of primers were 77.19 and 95.22%, respectively. There were significant genetic diversities among different ploidy levels of S. spontaneum. The coefficient of gene differentiation (Gst) and gene flow (Nm) of different ploidy levels of S. spontaneum were 0.31 and 1.11, respectively, suggesting that there is significant genetic differentiation among S. spontaneum from different geographical sources and that gene exchanges have occurred among S. spontaneum with different ploidy levels. Cluster analysis showed that S. spontaneum of the same ploidy level tended to cluster together, and variance analysis showed that intra-specific variation was significantly higher than inter-specific variation in S. spontaneum with different ploidy levels. The results showed that S. spontaneum from Tibet China, Nepal and Myanmar demonstrates significant genetic diversity and that gene exchanges have occurred among S. spontaneum with different ploidy levels. The ploidy evolution of germplasms might be a mesh-type development rather than a single direction.


Similar content being viewed by others
References
Al-Somain, B.H.A., H.M. Migdadi, S.A. Al-Faifi, S.S. Alghamdi, A.A. Muharram, N.A. Mohammed, and Y.A. Refay. 2017. Assessment of genetic diversity of sesame accessions collected from different ecological regions using sequence-related amplified polymorphism markers. 3 Biotech 7(1): 82. https://doi.org/10.1007/s13205-017-0680-2.
Aitken, K., J.H. Li, G. Piperidis, Q. Cai, Y.H. Fan, and P. Jackson. 2018. Worldwide genetic diversity of the wild species Saccharum spontaneum and level of diversity captured within sugarcane breeding programs. Crop Science 58: 218–229.
Alwala, S., A. Suman, J.A. Arro, J.C. Veremis, and C.A. Kimbeng. 2006. Target region amplification polymorphism (TRAP) for assessing genetic diversity in sugarcane germplasm collections. Crop Science 46(1): 448–455.
Bergstrom, I. 1938. Tetraploid apple seedlings obtained from the progeny of triploid varieties. Hereditas 24(1–2): 210–215. https://doi.org/10.1111/j.1601-5223.1938.tb03215.x.
Bielig, L.M., A. Mariani, and N. Berding. 2003. Cytological studies of 2n male gamete formation in sugarcane, Saccharum L. Euphytica 133(1): 117–124. https://doi.org/10.1023/A:1025628103101.
Bingham, E.T. 1968. Aneuploids in seedling populations of tetraploid alfalfa, Medicago sativa L. Crop Science 8(5): 571–574.
Budak, H., R.C. Shearman, I. Parmaksiz, R.E. Gaussoin, T.P. Riordan, and I. Dweikat. 2004. Molecular characterization of buffalograss germplasm using sequence-related amplified polymorphism markers. Theoretical and Applied Genetics 108(2): 328–334. https://doi.org/10.1007/s00122-003-1428-4.
Chen, X.W., H.H. Deng, and Y.S. Chen. 2010. Utilization of Badila in the breeding of YC-series parents and new varieties of sugarcane. Sugarcane and Canesugar 6: 1–5.
Cheng, B.Y., J.H. Xia, J.Y. Gong, and S.H. Yang. 2011. Application of capillary electrophoresis detection with fluorescent SSR markers in rice DNA fingerprint identification. Chinese Journal of Rice Science 25(6): 672–676.
Cheng, R.K. 2010. Modern sugarcane genetic breeding. Beijing: China Agriculture Press.
Chandra, A., M.P. Grisham, and Y.B. Pan. 2014. Allelic divergence and cultivar-specific SSR alleles revealed by capillary electrophoresis using fluorescence-labeled SSR markers in sugarcane. Genome 57(6): 363–372. https://doi.org/10.1139/gen-2014-0072.
Chang, D., F.Y. Yang, J.J. Yan, Y.Q. Wu, S.Q. Bai, X.Z. Liang, Y.W. Zhang, and Y.M. Gan. 2012. SRAP analysis of genetic diversity of nine, native populations of wild sugarcane, Saccharum spontaneum, from Sichuan, China. Genetics and Molecular Research 11(2): 1245–1253. https://doi.org/10.4238/2012.May.9.3.
D’Hont, A., D. Ison, K. Alix, C. Roux, and J.C. Glaszmann. 1998. Determination of basic chromosome numbers in the genus Saccharum by physical mapping of ribosomal RNA genes. Genome 41(2): 221–225. https://doi.org/10.1139/g98-023.
D’Hont, A. 2005. Unraveling the genome structure of polyploids using FISH and GISH; examples of sugarcane and banana. Cytogenetic and Genome Research 109(1–3): 27–33. https://doi.org/10.1159/000082378.
Dujardin, M., and W.W. Hanna. 1988. Cytology and breeding behavior of a partially fertile triploid pearl millet. Journal of Heredity 79(3): 216–218. https://doi.org/10.1093/oxfordjournals.jhered.a110499.
Gu, X.Y., Z.H. Guo, X.Q. Zhang, Y.H. Zhou, S.Q. Bai, C.B. Zhang, Z.R. Jiang, X. Liu, C.J. Zhou, and X. Ma. 2014. Genetic diversity of Elymus sibiricus germplasm resources revealed by SRAP markers. Acta Prataculturae Sinica 23(1): 205–216.
Hu, C.M. 2015. Investigation, collection and cytological identification of wild sugarcane germplasm resources of southern Tibet. Master diss., Yunnan Agricultural University, Kunming, Yunnan.
Hamrick, J.L., and M.J.W. Godt. 1996. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions: Biological Sciences 351(1345): 1291–1298. https://doi.org/10.1098/rstb.1996.0112.
He, S.C., Q.H. Yang, F.H. Xiao, F.C. Zhang, and L.L. He. 1999. Collections and description of basic germplasm of sugarcane (Saccharum complex) in China. International Sugar Journal Worldwide Sugar Edition 101(1201): 84–93.
Hornsey, K.G. 1973. The occurrence of hexaploid plants among autotetraploid populations of sugar beet, (Beta Vulgaris L.) and the production of tetraploid progeny using a diploid pollinator. Caryologia 26(2): 225–228. https://doi.org/10.1080/00087114.1973.10796538.
Howard, H.W. 1939. The size of seeds in diploid and autotetraploid Brassica oleracea L. Journal of Genetics 38 (1–2): 325–340. https://doi.org/10.1007/BF02982177.
Li, S.J. 2017. Cytological identification of Saccharum spontaneum L. resources from Myanmar based on the differences of karyotype and ploidy. Master diss., Yunnan Agricultural University, Kunming, Yunnan.
Liao, S.T. 2012. Assessment of cold tolerance in different sugarcane varieties using SRAP markers. Master diss., Guangxi University, Nanning, Guangxi.
Lammerts, W.E. 1931. Interspecific hybridization in Nicotiana. XII. The amphidiploid rustica-paniculata hybrid: Its origin and cytogenetic behavior. Genetics 16(3): 191–211.
Lange, W., and M. Wagenvoort. 1973. Meiosis in triploid Solanum tuberosum L. Euphytica 22(1): 8–18. https://doi.org/10.1007/BF00021550.
Li, G., and C.F. Quiros. 2001. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in brassica. Theoretical and Applied Genetics 103(2–3): 455–461. https://doi.org/10.1007/s001220100570.
Liu, X.L., X.J. Li, C.H. Xu, X.Q. Lin, and Z.H. Deng. 2016. Genetic diversity of populations of Saccharum spontaneum, with different ploidy levels using SSR molecular markers. Sugar Tech 18(4): 1–8. https://doi.org/10.1007/s12355-015-0399-5.
Mehra, P.N., and O.P. Sood. 1974. Floating chromosomal populations in Saccharum spontaneum L. Cytologia 39: 681–696.
Mok, D.W.S., S.J. Peloquin, and T.R. Tarn. 1975. Cytology of potato triploids producing 2n pollen. American Potato Journal 52(6): 171–174. https://doi.org/10.1007/BF02838107.
Norrmann, G.A., C.L. Quarín, and K.H. Keeler. 1997. Evolutionary implications of meiotic chromosome behavior, reproductive biology, and hybridization in 6 × and 9 × cytotypes of Andropogon gerardii (Poaceae). American Journal of Botany 84(2): 201–207.
Peng, S.G. 1990. Sugarcane breeding. Beijing: China Agricultural Press.
Pan, Y.B., B.E. Scheffler, and E.P. Richard Jr. 2007. High throughput genotyping of commercial sugarcane clones with microsatellite (SSR) DNA markers. Sugar Tech 9(2–3): 176–181.
Panje, R.R., and C.N. Babu. 1959. Studies in Saccharum spontaneum distribution and geographical association of chromosome numbers. Cytologia 25(2): 152–172.
Price, S. 1959. Cytological studies in saccharum and allied genera V. chromosome numbers in Saccharum, Erianthus, Narenga, and Sclerostachya from Thailand and Vietnam. Cytologia 24: 342–346.
Ramsey, J., and D.W. Schemske. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics 29(1): 467–501.
Rick, C.M. 1945. A survey of cytogenetic causes of unfruitfulness in the tomato. Genetics 30(4): 347–362.
Rod, P., and S. Petere. 2005. Genalex 6: genetic analysis in excel. Population genetic software for teaching and research. Molecular Ecology Notes 6: 288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x.
Song, X.X. 2009. Genetic diversity sugarcane and its relatives by SRAP and TRAP markers. Master diss., Fujian Agriculture and Forestry University, Fujian.
Sreenivasan, T.V. 1975. Cytogenetical studies in Saccharum spontaneum. Proceedings of the Indian Academy of Sciences - Section B 81(3): 131–144. https://doi.org/10.1007/BF03050754.
Sreenivasan, T.V., and D. Jagathesan. 1975. Meiotic abnormalities in Saccharum spontaneum. Euphytica 24(2): 543–549. https://doi.org/10.1007/BF00028230.
Sheji, M., N.V. Nair, C. Pankajkumar, and A. Selvi. 2006. Analysis of genetic diversity among Saccharum spontaneum L. from four geographical regions of India, using molecular markers. Genetic Resources and Crop Evolution 53(6): 1221–1231. https://doi.org/10.1007/s10722-005-2433-z.
Tyagi, B.R. 1988. The mechanism of 2n pollen formation in diploids of Costus speciosus (Koenig) J. E. Smith and role of sexual polyploidization in the origin of intraspecific chromosomal races. Cytologia 53: 763–770.
Tew, T.L., and Y.B. Pan. 2010. Microsatellite (simple sequence repeat) marker-based paternity analysis of a seven-parent sugarcane polycross. Crop Science 50(4): 1401–1408.
Wang, S.Q., Z.L. Wang, C.F. Guo, S.M. Guo, and D.H. Zeng. 1996. Studies on the chromosome of Saccharum spontaneum from Fujian. Sugarcane and Canesugar 5: 9–13.
Wen, J.C., Q. Cai, Y.H. Fan, M. Zhang, and H. Cheng. 2001. Studies on the chromosome numbers of Saccharum spontaneum and related plants-sclerostachya, Narenga in China. Sugarcane and Canesugar 3: 12–15.
Wright, S. 1978. Evolution and the genetics of populations vol 4: Variability within and among natural populations. Chicago: University of Chicago Press.
Yang, Q.H., and S.C. He. 1996. Studies on Saccharum spontaneum chromosome numbers and geographical distribution Yunnan, China. Sugarcane 3(1): 10–13.
You, Q., Y.B. Pan, L.P. Xu, S.W. Gao, Q.N. Wang, Y.C. Su, Y.Q. Yang, Q.B. Wu, D.G. Zhou, and Y.X. Que. 2016. Genetic diversity analysis of sugarcane germplasm based on fluorescence-labeled simple sequence repeat markers and a capillary electrophoresis-based genotyping platform. Sugar Tech 18(4): 380–390. https://doi.org/10.1007/s12355-015-0395-9.
Acknowledgements
The research described in here was supported by the National Science Foundation of China (31260348, 31460372 and 31760417).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, XH., Wang, XH. & Yang, QH. Genetic Diversity and Phylogenetic Relationship of Saccharum spontaneum L. with Different Ploidy Levels Based on SRAP Markers. Sugar Tech 21, 802–814 (2019). https://doi.org/10.1007/s12355-019-00700-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00700-5