Abstract
Due to the progressive nature of type 2 diabetes (T2D), the majority of patients require increasing levels of therapy to achieve and maintain good glycemic control. At present, once patients become uncontrolled on oral antidiabetic therapies, the two primary treatment options are glucagon-like peptide-1 receptor agonists (GLP-1RAs) or basal insulin, although earlier use of GLP-1RAs has also been advocated. While both of these drug classes have proven efficacy in treating T2D, there can be limitations to their use in some patients, and resistance to further treatment intensification among both patients and physicians. More recently, treatment incorporating both a GLP-1RA and a basal insulin has been used successfully in the clinic and the first such combination product, IDegLira (insulin degludec + liraglutide), has recently been approved for use in Europe. IDegLira combines insulin degludec and the GLP-1RA liraglutide in a single injection. In both insulin-naïve and basal insulin-treated individuals with T2D, IDegLira has demonstrated greater reductions in glycated hemoglobin (HbA1c) than either of the individual components, with a low rate of hypoglycemia and weight loss. IDegLira may provide a new option for patients requiring treatment intensification but for whom increased weight or a higher risk of hypoglycemia are barriers. This article discusses the rationale behind combining these two drug classes and reviews the available clinical evidence for the efficacy and safety of IDegLira.
Similar content being viewed by others
Introduction: The Need for Treatment Intensification
Diabetes mellitus is a growing global epidemic with a serious impact on healthcare systems and economic costs. In 2013, there were an estimated 382 million people living with diabetes worldwide and a total global healthcare expenditure of US$548 billion related to treating the disease [1]. Further, diabetes prevalence is increasing, with the number of people with diabetes predicted to rise to 592 million by 2035 [1]. The incidence of type 2 diabetes (T2D) in particular is on the increase and expected to make up the majority of new cases of diabetes diagnosed between now and 2035 [1].
Current treatment of T2D focuses on achieving tight glycemic control to minimize long-term microvascular complications, namely retinopathy, nephropathy, and neuropathy. Tight glycemic control achieved with intensive glucose-lowering treatment within the first years after diagnosis reduces the risk of long-term complications of diabetes, resulting in improved quality of life for the patient and decreased healthcare costs [2]. However, there is a need to exercise judgment in determining who should receive treatment aimed at achieving stringent glycemic targets. In those patients with coronary disease, renal failure and advanced age, a more relaxed treatment target may be more appropriate [2, 3].
In combination with fasting plasma glucose (FPG) and postprandial glucose, measurement of glycated hemoglobin (HbA1c) is the usual method of diagnosing and clinically tracking diabetes control. Current guidelines for the treatment of T2D recommend that patients should aim for glycemic targets ranging from HbA1c <7.5% (National Institute for Health and Care Excellence [NICE]) and <7.0% (American Diabetes Association [ADA] and European Association for the Study of Diabetes [EASD]) to ≤6.5% (American Association of Clinical Endocrinologists [AACE]), with the need for intensive versus more relaxed control based on multiple factors such as age and the presence of comorbidities [2, 4–6]. Unfortunately, a large proportion of people with T2D globally are not currently meeting these targets [7–9].
There are limitations to the use of HbA1c as a diagnostic measure, particularly early in T2D disease progression, as some studies have found evidence of diabetic complications such as proliferative retinopathy at HbA1c levels <6.5% [10, 11]. Patients may be labeled as having prediabetes if their HbA1c is <6.5% but they have certain risk factors or comorbidities such as obesity, dyslipidemia, or a family history of diabetes. Unfortunately, there are currently no pharmacological agents approved for the management of prediabetes, and patients must rely initially on lifestyle measure alone. However, the AACE guidelines do list metformin as a first-line drug in prediabetes, but also allow glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) as appropriate therapy in these patients when diet and exercise alone are not successful (although this is currently off-label) [6].
Good glycemic control is further complicated by the progressive nature of T2D. The majority of patients will require continual intensification of treatment as beta cell function deteriorates and endogenous insulin production declines [2]. The majority of available antidiabetic therapies lack sustainability of glycemic control, suggesting further progressive beta cell deterioration despite their use, and further necessitating treatment intensification [2]. As such, early identification of T2D, particularly in high-risk individuals, may be justified, along with earlier initiation of pharmacotherapy aimed at preserving beta cell function and minimizing long-term microvascular complications.
Current Treatment Options
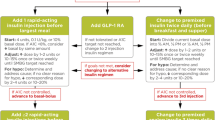
The first line of treatment in T2D is lifestyle and diet modification; in most instances, this is then followed by initiation of treatment with metformin if blood glucose levels remain uncontrolled. Most guidelines then recommend adding in further oral antidiabetics (primarily a sulphonylurea [SU] or thiazolidinedione), dipeptidyl peptidase-4 (DPP-4) inhibitors, GLP-1RAs, sodium–glucose co-transporter 2 (SGLT-2) inhibitors or (last but not least) insulin after 3 months if target HbA1c is not achieved and depending on factors such as the patients’ body mass index and relative hypoglycemia risk [3]. In our hypothetical patient (see “Hypothetical Clinical Case: Background”), the addition of an SU as add-on to metformin resulted in minor hypoglycemia and weight gain.
The AACE guidelines in particular emphasize the need to minimize weight gain and risks of hypoglycemia, and to stratify treatment recommendations according to HbA1c after failure of lifestyle modifications. In patients with HbA1c <7.5% prior to initiation of antidiabetic agents, metformin is recommended as first-line therapy. However, in patients with HbA1c ≥7.5% in conjunction with metformin, either DPP-4 inhibitors, SGLT-2 inhibitors, or GLP-1RAs are recommended as therapy intensification. Preference is given to GLP-1RAs because of their potent effect on HbA1c and/or weight loss. In patients with HbA1c >9%, either dual or triple therapy or immediate initiation of insulin is recommended [6]. In all guidelines, initiation of basal insulin is indicated if the patient fails to reach or maintain glycemic targets on a combination of two or more antidiabetic agents [3, 6].
There is increasing focus on the need to individualize therapy and targets based on the particular needs of the patient [3]. In particular, there have been calls to consider earlier initiation of GLP-1RAs (with further intensification using basal insulin if required) in the treatment pathway [3], with the possibility that this might slow disease progression and preserve some pancreatic function in some patients. In support of this approach are clinical data showing improvements in measures of beta cell function such as homeostasis model assessment-B (HOMA B) and proinsulin-to-insulin ratio with GLP-1RAs [12–14]. In addition to consideration of beta cell function, the risk of hypoglycemia should also be taken into account. Specifically, in those at particular risk of hypoglycemia, a GLP-1RA may be preferable to an SU due to the glucose-dependent action of the former versus the glucose-independent insulin secretion caused by the latter.
The choice between addition of a GLP-1RA and immediate initiation of a basal insulin depends on the degree of disease progression, the level of glycemic control and other factors such as the risk of weight gain, each of which will be specific to each individual patient. The two address different portions of the pathophysiological deficits in T2D, and each can safely and effectively help many patients to achieve recommended glucose targets when they are no longer able to do so with lifestyle modification and oral antidiabetic drugs (OADs) alone [15–19].
GLP-1 Receptor Agonists
GLP-1RAs have several benefits compared with basal insulin therapy in people with T2D who retain a level of endogenous insulin secretion. Due to their glucose-dependent mechanism of action, long-acting GLP-1RAs (e.g., liraglutide, albiglutide, dulaglutide, exenatide extended release) address both postprandial and fasting blood glucose, in contrast to basal insulin, which is designed to offer fasting blood glucose control and inter-meal control only, and short-acting GLP-1RAs such as exenatide and lixisenatide, which offer more prandial control and lower fasting control due to their short half-life. The glucose-dependent action of these agents also entails a lower risk of hypoglycemia compared with basal insulin [17, 18].
Additionally, it is well established that GLP-1RAs encourage weight loss via extra-pancreatic effects such as slowing gastric emptying and reducing appetite at the level of the hypothalamus, resulting in diminished energy intake [17–20]. Weight loss of as much as 3 kg over 52 weeks has been demonstrated with GLP-1RAs, with liraglutide demonstrating the greatest weight loss to date [21], while OADs, particularly SUs and thiazolidinediones, show a consistent tendency toward weight gain over time [2, 22].
Across many clinical trials, the GLP-1RAs have been shown to be effective in all stages of diabetes. However, in those patients with little to no beta cell function, initiation of basal insulin is a necessary next step to reach and maintain glycemic targets. Initiation of the GLP-1RA liraglutide is described as ‘option 1’ for our hypothetical patient.
Hypothetical Clinical Case Option 1: Addition of Liraglutide
After discussion about the potential adverse events and side effects that can be experienced, Lewis agrees to the addition of liraglutide. He is started at a dose of 0.6 mg daily for 1 week and, on week 2, titrates up to 1.2 mg daily. During this titration phase, glimepiride is discontinued. He complains about early satiety and eating less. At one point, he wanted to “get his money’s worth” from a meal so forced himself to finish his meal. This precipitated some nausea followed by vomiting. The symptoms went away as time passed, and eventually he titrated to a dose of 1.8 mg daily. He managed to lose 12 pounds (5.4 kg) over the course of 3 months, and his glycated hemoglobin (HbA1c) decreased to 7.2%. He has had hardly any hypoglycemic events; however, he does note at times that his mid-afternoon glucose is in excess of 200 mg/dL (11.1 mmol/L). Intensification of his therapy is discussed during this visit and he commits to being more engaged in the vigorous exercise program recommended, to improve insulin sensitivity and assist in weight loss.
Basal Insulin
The efficacy of basal insulin in T2D is well established [4]. However, basal insulin has traditionally been the final choice of treatment in T2D, initiated only when the patient is unable to maintain good glycemic control after all previous options have been tried [3].
The newer basal insulin analogs, molecularly designed to have specific pharmacokinetic properties, have demonstrated significant improvements over earlier insulins such as neutral protamine Hagedorn (NPH) in terms of day-to-day variability, effects on weight and risks of hypoglycemia [23–27]. Improvements in day-to-day variability of glucose-lowering effect are of particular note as it has been shown that greater fluctuation in FPG is linked to higher levels of mortality [28]. Insulin detemir, insulin glargine, and insulin degludec all demonstrate decreased intra-patient variability compared with NPH insulin [23, 29], while insulin degludec has also shown decreased variability compared with insulin glargine [23]. This decreased variability leads to a more predictable action and so a decreased risk of hypoglycemia compared with insulin glargine including a reduction of up to 36% in nocturnal hypoglycemic events [27, 30].
Unfortunately, reluctance to initiate insulin therapy persists even when patients fail to meet glycemic targets on multiple OADs and there is evidence that physicians continue to delay initiation of basal insulin despite prolonged HbA1c levels [31]. In one study of inertia, patients on two OADs and with HbA1c >8% experienced a mean delay of 26 months prior to insulin initiation; in patients with HbA1c between 7% and 8%, the delay was 51 months [31]. In addition, many patients do not reach glycemic targets (HbA1c ≤7.0%) with basal insulin, either with a treat-to-target approach in clinical trials [32–34] or in the general clinic [35]. Initiation of basal insulin degludec is described as ‘option 2’ for our hypothetical patient.
Hypothetical Clinical Case Option 2: Addition of a Basal Insulin Analog
Insulin degludec is initiated at a starting dose of 10 units at bedtime. Lewis titrated by 3 units every 3 days using a self-titration algorithm, and managed to reach a fasting plasma glucose (FPG) target of approximately 100 mg/dL (5.6 mmol/L). His other medications remain the same. He has suffered two minor nocturnal hypoglycemic events over the past 2 weeks, but it appears that his daytime control is a little better. (Continuous glucose monitoring data can be incorporated and it is important to note that nocturnal hypoglycemic events have occurred in patients using basal insulin analogs with near-normal glycated hemoglobin [HbA1c].) Furthermore, the nocturnal hypoglycemic events often went unnoticed. His HbA1c today is 6.9%, but he has gained an additional 6 pounds (2.7 kg).
Challenges and Barriers to Intensive Treatment
Although the benefits of intensive therapy in delaying the onset of diabetic complications are well established, numerous studies have shown that intensive glucose control, particularly with agents such as insulin and SUs, can result in an increased risk of hypoglycemia and substantial weight gain [2, 36–38]. Fear of these negative side effects can lead to both patients and physicians being reluctant to intensify therapy, particularly with insulin [39–41]. In addition, fear of and experience of both hypoglycemia and weight gain can negatively affect adherence to therapy [40], which in turn has an impact on long-term glycemic control [42]. The fear of and experience of hypoglycemia may also lead to de-escalation of insulin therapy in some patients [43]. Conversely, there is some evidence suggesting that patients who lose weight on their diabetes therapy show better treatment adherence than those who gain weight during treatment [44].
Once basal insulin has been initiated, a further barrier to intensification is the increased number of injections and the increased regimen complexity necessitated by the addition of prandial insulin injections to basal therapy [40, 41].
Because of the increased risks of hypoglycemia and weight gain, and the likelihood of decreased adherence as these risks increase, treatment guidelines currently recommend less stringent treatment, with individualized targets and higher glycemic targets in patients at particular risk of hypoglycemia, of advanced age, with multiple comorbidities and in those patients whose adherence to treatment is lower [3].
While more recently introduced basal insulin analogs demonstrate less variability than NPH, leading to a reduced risk of hypoglycemia [30] and a greater potential for patients to confidently self-titrate [45], there is still a pronounced fear of these side effects among patients [39, 40]. Sometimes, primary care physicians are also reluctant to prescribe injectable therapies due to a lack of education and/or the time-consuming nature of training and follow-up of patients initiating insulin therapy [40, 46]. Patient perception of failure to control their diabetes, fear, embarrassment or inconvenience of injection(s), and cost of therapies are other potential barriers to insulin initiation [40, 46, 47].
GLP-1RAS and Basal Insulin: Rationale for a New Combination Therapy
As outlined above, while the current options available for post-OAD therapy in T2D have proven efficacy, they are not ideal for all patients. This is particularly the case for patients who require more intensive treatment to meet glycemic targets but who are at risk of significant weight gain or hypoglycemia. Basal insulins and GLP-1RAs have complementary modes of action in the treatment of T2D. As such, there is great interest in the potential use of these agents in combination for some patients who require greater reductions in HbA1c [48–51].
The feasibility of adding either a GLP-1RA to basal insulin therapy or a basal insulin analog to GLP-1RA therapy has been tested in several trials in which a potential for greater HbA1c reductions than with either therapy alone has been demonstrated [52–54].
In one such trial, 988 participants uncontrolled on metformin with or without SU discontinued SU and started on liraglutide, titrated up to 1.8 mg, for a 12-week run-in period. At the end of this run-in period, those who had not reached HbA1c <7% were randomized to either add-on insulin detemir or continue on liraglutide plus metformin for 26 weeks. Post-randomization, addition of insulin detemir led to a further reduction in HbA1c of 0.5% (from 7.6% at randomization) compared with a 0.02% increase in HbA1c with continued liraglutide plus metformin alone [52].
In a study of liraglutide versus insulin aspart as add-on to basal insulin degludec, addition of liraglutide led to a significantly greater reduction in HbA1c (−0.74%) at 26 weeks than did once-daily prandial insulin aspart (−0.39%) with a treatment difference of −0.32% (95% CI −0.53 to −0.12, P = 0.0024) [53]. Further to this improvement in HbA1c, significant reduction in weight and a reduced risk of hypoglycemia was demonstrated when compared with intensification by addition of prandial insulin to basal insulin therapy [53].
Due to the distinct, stable molecular forms of both insulin degludec and liraglutide and their complementary modes of action, IDegLira was developed. Granted marketing authorization in the European Union as of September 2014, IDegLira is the first combination of a basal insulin (insulin degludec) and a GLP-1 analog (liraglutide) in one pen. Also under development is a lixisenatide and insulin glargine combination, although phase 3 trials are still ongoing and, at present, limited clinical data are available for this product.
IDegLira is a fixed ratio of insulin degludec (100 U/mL) and liraglutide (3.6 mg/mL) with a maximum dose of 50 Units IDeg/1.8 mg liraglutide, corresponding with the maximum approved dose of liraglutide, where the unit of measure for this fixed-ratio combination will be noted as ‘dosing steps’. The combination has the potential to provide improved overall glycemic control whilst mitigating some of the common side effects experienced with GLP-1RAs and basal insulin (e.g., nausea, weight gain, and hypoglycemia).
IDegLira: Clinical Evidence
At present, published data are available for two phase 3 clinical trials of IDegLira, one in insulin-naïve patients and one in patients previously treated with basal insulin. Both were 26-week (one with a further 26-week extension phase [55]) randomized trials (2:1:1 and 1:1, respectively), the first (DUAL I; ClinicalTrials.gov number, NCT01336023) being a treat-to-target, open-label study comparing IDegLira with insulin degludec or liraglutide alone in insulin-naïve patients previously treated with metformin with or without pioglitazone [55]. The second study (DUAL II; ClinicalTrials.gov number, NCT01392573) was a double-blind trial of IDegLira compared with insulin degludec in patients previously treated with basal insulin. As part of the study design in DUAL II, the degludec comparator arm was capped at 50 dose units. This was so that the relative contribution of the liraglutide component towards the overall efficacy of IDegLira could be judged more clearly, and was a regulatory requirement from the US Food and Drug Administration [56].
In terms of efficacy in insulin-naïve patients, treatment with IDegLira produced a significantly greater reduction in HbA1c (−1.9% from baseline) than either degludec (−1.4% from baseline, estimated treatment difference [ETD] −0.5%, 95% CI −0.6 to −0.4, P < 0.0001) or liraglutide (−1.3% from baseline, ETD −0.6%, 95% CI −0.8 to −0.5, P < 0.0001) alone after 26 weeks [56]. In addition, a significantly greater proportion of patients achieved glycemic targets of HbA1c <7% after 26 weeks of treatment with IDegLira than with degludec (81% vs. 65%, P < 0.0001) or liraglutide (60%, P < 0.0001) and HbA1c <6.5% compared with degludec (70% vs. 47%, P < 0.0001) or liraglutide (70% vs. 41%, P < 0.0001).
This improvement in glycemic control occurred in conjunction with a mean body weight reduction of −0.5 kg with IDegLira, compared with a weight increase of 1.6 kg with degludec (P < 0.0001 vs. IDegLira) and a weight loss of 3.0 kg with liraglutide. In addition, IDegLira also demonstrated a 32% lower rate of hypoglycemia than degludec despite a lower end-of-trial HbA1c (6.4% vs. 6.9% [46 mmol/mol vs. 52 mmol/mol]). As would be expected due to its mode of action, few subjects reported hypoglycemia with liraglutide [55].
In those previously treated with basal insulin, patients receiving IDegLira experienced a significantly greater reduction in HbA1c compared with those on degludec (capped at 50 Units) after 26 weeks (−1.9% vs. −0.9%, P < 0.0001) [56]. At the 26-week endpoint, 60% of participants in the IDegLira group had achieved HbA1c <7% versus 23% in the degludec arm (P < 0.0001) and a significantly higher proportion (40%) of patients in the IDegLira arm achieved HbA1c <7% with no confirmed hypoglycemic episodes during the last 12 weeks of treatment and with no weight gain, than in the degludec group (8.5%, P < 0.0001).
In this trial, patients receiving IDegLira experienced a mean weight loss of 2.7 kg compared with no weight change with degludec. Confirmed hypoglycemia (including severe events and defined as plasma glucose <56 mg/dL [3.1 mmol/L] regardless of symptoms, or if assistance required) was not statistically significantly lower than for degludec (1.5 events/patient-year vs. 2.6 events/patient-year; P = not significant) with similar incidences (IDegLira 24% vs. degludec 25%) and lower HbA1c with IDegLira [56].
IDegLira was well tolerated in both trials, with comparable levels of adverse events to the individual treatment arms and low incidence of severe adverse events [55, 56]. Overall, the incidence of nausea was higher in the IDegLira group than in the degludec group in both trials (9% vs. 4% of patients in DUAL I; 6.5% vs. 3.5% in DUAL II). However, in DUAL I, the incidence of nausea was lower with IDegLira than with liraglutide (9% vs. 20% patients). This reduced level of nausea with IDegLira compared with liraglutide is of particular interest and likely stems from the more gradual increase in dose of liraglutide when initiating and titrating IDegLira compared with the standard liraglutide titration.
Overall, IDegLira offers simple titration of two efficacious therapies in a single daily injection while mitigating the principal side effects of basal insulin (hypoglycemia and weight gain) and GLP-1RA (nausea) when given alone [55, 56]. Using a GLP-1RA and basal insulin together in two separate injections can provide the greater dosing flexibility that some patients may require (such as those in need of high insulin doses), but having both agents in one pen will offer greater convenience/simplicity and may reduce patient confusion. IDegLira will also offer a new weight-neutral option for insulin initiation in patients uncontrolled on OADs that has a lower risk of hypoglycemia versus basal insulin initiation [55]. Initiation of IDegLira is described as ‘option 3’ for our hypothetical patient.
Conclusions
There are a number of treatment options available for consideration when intensifying treatment in patients with T2D, and there are many factors to take into account when deciding how to best achieve treatment goals. Treatment should always be individualized to most closely meet the needs and preferences of the patient.
GLP-1RAs such as liraglutide demonstrate postprandial glucose control as well as fasting glucose control due to suppression of glucagon release, both in a glucose-dependent fashion. In contrast, basal insulins such as insulin degludec have been shown to offer superior FPG control as well as inter-meal control. IDegLira is the first fixed-ratio combination of a basal insulin and GLP-1 analog in a single injection and this novel combination incorporates glucose-dependent prandial control coupled with the augmentation of fasting and inter-meal control offered by insulin degludec. In clinical trials to date, IDegLira has demonstrated improved HbA1c in patients with T2D compared with either liraglutide or insulin degludec alone, and with a lower risk of hypoglycemia and weight gain than insulin degludec alone. As such, IDegLira offers another option for patients and physicians who may be reluctant to initiate or intensify insulin therapy due to concerns about hypoglycemia and weight gain.
References
IDF Diabetes Atlas 6th Edition, 2013. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf. Last accessed Nov, 2014.
UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Nathan DM, Buse JB, Davidson MB, American Diabetes Association, European Association for Study of Diabetes, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
National Institute for Health and Care Excellence. The management of type 2 diabetes: NICE clinical guideline 87. 2009. Available from: http://www.nice.org.uk/guidance/cg87. Last accessed Aug, 2014.
Garber AJ, Abrahamson MJ, Barzilay JI, American Association of Clinical Endocrinologists, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–36.
Lopez Stewart G, Tambascia M, Rosas Guzmán J, Etchegoyen F, Ortega Carrión J, Artemenko S. Control of type 2 diabetes mellitus among general practitioners in private practice in nine countries of Latin America. Rev Panam Salud Publica. 2007;22:12–20.
Oguz A, Benroubi M, Brismar K, et al. Clinical outcomes after 24 months of insulin therapy in patients with type 2 diabetes in five countries: results from the TREAT study. Curr Med Res Opin. 2013;29:911–20.
Liebl A, Jones S, Goday A, et al. Clinical outcomes after insulin initiation in patients with type 2 diabetes: 24-month results from INSTIGATE. Diabetes Ther. 2012;3:9.
Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Klein R, Klein BEK. Relation of glycemic control to diabetic complications and health outcomes. Diabetes Care. 1998;21(Suppl 3):C39–43.
Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–3.
Seino Y, Rasmussen MF, Clauson P, Kaku K. The once-daily human glucagon-like peptide-1 analog, liraglutide, improves β-cell function in Japanese patients with type 2 diabetes. J Diabetes Investig. 2012;3:388–95.
Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD). Diabetes Care. 2009;32:1224–30.
Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311:2315–25.
Holman RR, Thorne KI, Farmer AJ, 4-T Study Group, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–30.
Schwartz S. Evidence-based practice use of incretin-based therapy in the natural history of diabetes. Postgrad Med. 2014;126:66–84.
Russell S. Incretin-based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm. 2013;35:159–72.
Russell-Jones D, Gough S. Recent advances in incretin-based therapies. Clin Endocrinol (Oxf). 2012;77:489–99.
Horowitz M, Flint A, Jones KL, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:258–66.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Kahn SE, Haffner SM, Heise MA, ADOPT Study Group, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43.
Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–64.
Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2008;81:184–9.
Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13:1008–19.
Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84.
Rodbard HW, Gough S, Lane W, Korsholm L, Bretler DM, Handelsman Y. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized BEGIN trials. Endocr Pract. 2014;20:285–92.
Muggeo M, Zoppini G, Bonora E, et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45–50.
Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–20.
Vora J, Christensen T, Rana A, Bain SC. Insulin degludec versus insulin glargine in type 1 and type 2 diabetes: a meta-analysis of endpoints in phase 3a trials. Diabetes Ther. 2014;5:435–46.
Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535–40.
Blonde L, Merilainen M, Karwe V, Raskin P, TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11:623–31.
Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6.
Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–16.
Blak BT, Smith HT, Hards M, Maguire A, Gimeno V. A retrospective database study of insulin initiation in patients with Type 2 diabetes in UK primary care. Diabet Med. 2012;29:e191–8.
Patel A, MacMahon S, Chalmers J, ADVANCE Collaborative Group, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Gerstein HC, Miller ME, Byington RP, et al. ACCORD Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14:646–55.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682–9.
Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;164:6–10.
Aikens JE, Piette JD. Longitudinal association between medication adherence and glycaemic control in Type 2 diabetes. Diabet Med. 2013;30:338–44.
Munro N, Barnett AH. Incidence, worry and discussion about dosing irregularities and self-treated hypoglycaemia amongst HCPs and patients with type 2 diabetes: results from the UK cohort of the Global Attitudes of Patient and Physicians (GAPP2) survey. Int J Clin Pract. 2014;68:692–9.
Grandy S, Fox KM, Hardy E, SHIELD Study Group. Association of weight loss and medication adherence among adults with type 2 diabetes mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes). Curr Ther Res Clin Exp. 2013;75:77–82.
Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9:902–13.
Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62:860–8.
Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33:733–5.
Holst JJ, Vilsboll T. Combining GLP-1 receptor agonists with insulin: therapeutic rationales and clinical findings. Diabetes Obes Metab. 2013;15:3–14.
Meneghini LF. Intensifying insulin therapy: what options are available to patients with type 2 diabetes? Am J Med. 2013;126(9 Suppl 1):S28–37.
Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485–502.
Vora J, Bain SC, Damci T, et al. Incretin-based therapy in combination with basal insulin: a promising tactic for the treatment of type 2 diabetes. Diabetes Metab. 2013;39:6–15.
DeVries JH, Bain SC, Rodbard HW, Liraglutide-Detemir Study Group, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35:1446–54.
Mathieu C, Rodbard HW, Cariou B, BEGIN: VICTOZA ADD-ON (NN1250-3948) study group, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16:636–44.
Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16:396–402.
Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–93.
Buse J, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926–33.
Acknowledgments
The article processing charges and open access fee for this publication were funded by Novo Nordisk A/S, Søborg, Denmark. Writing assistance in the preparation of this manuscript was provided by Claire Passmore, and editorial/submission assistance by Daria Renshaw, both of Watermeadow Medical. This assistance was funded by Novo Nordisk A/S. The sponsor reviewed the manuscript for medical accuracy prior to submission but was not otherwise involved in identifying data for inclusion or in drafting and revising the paper. Both named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Both authors were involved in deciding on the structure and all content for this article. Both authors also reviewed the manuscript outline and all drafts of the paper, suggested additional content and provided comments on the structure and content. Javier Morales provided the case study details for inclusion in the paper.
Conflict of interest
Dr. Javier Morales has received honoraria for participation in advisory board meetings for Novo Nordisk, Sanofi Aventis, Eli Lilly, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals. He is also on the speakers’ bureau for Novo Nordisk, and has received research Grants from Novo Nordisk, and Bristol-Myers Squibb. Dr. Ludwig Merker was a consultant and/or speaker for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme and Novo Nordisk, without any direct financial benefit.
Compliance with ethics guidelines
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by either of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Morales, J., Merker, L. Minimizing Hypoglycemia and Weight Gain with Intensive Glucose Control: Potential Benefits of a New Combination Therapy (IDegLira). Adv Ther 32, 391–403 (2015). https://doi.org/10.1007/s12325-015-0208-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0208-2