Abstract
Introduction
In 2012, the Advisory Committee on Immunization Practices (ACIP) revised recommendations for adult pneumococcal vaccination to include a sequential regimen of 13-valent pneumococcal conjugate vaccine (PCV13) followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23) for certain high-risk adults with immunocompromising conditions. This study, from a payer perspective, examined: (1) the cost-effectiveness of the new 2012 ACIP vaccine policy recommendation relative to the 1997 ACIP recommendation; (2) the cost-effectiveness of potential future pneumococcal vaccination policies; and (3) key assumptions that influence study results.
Methods
A static cohort model that incorporated costs, health outcomes, and quality-adjusted life-year (QALY) losses associated with invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia (NBPP) was developed to evaluate seven pneumococcal vaccination strategies for a 50-year-old adult cohort over a 50-year period using incremental cost-effectiveness ratios (ICERs).
Results
For objective 1, the 2012 ACIP recommendation is the more economically efficient strategy (ICER was $25,841 per QALY gained vs. no vaccination). For objective 2, the most efficient vaccination policy would be to maintain the 2012 recommendation for PPSV23 for healthy and immunocompetent adults with comorbidities, and to modify the recommendation for adults with immunocompromising conditions by replacing PPSV23 with a sequential regimen of PCV13 and PPSV23 at age 65 (ICER was $23,416 per QALY gained vs. no vaccination). For objective 3, cost-effectiveness ratios for alternative pneumococcal vaccine policies were highly influenced by assumptions used for vaccine effectiveness against NBPP and accounting for the herd protection effects of pediatric PCV13 vaccination on adult pneumococcal disease.
Conclusion
Modifying the 2012 recommendation to include an additional dose of PCV13 at age 65, followed by PPSV23, for adults with immunocompromising conditions appears to be a cost-effective vaccine policy. Given the uncertainty in the available data and the absence of key influential data, comprehensive sensitivity analyses should be conducted by policy-makers when evaluating new adult pneumococcal vaccine strategies.
Similar content being viewed by others
Introduction
Of the two pneumococcal vaccines currently approved for use in adults in the United States (US), Europe, and selected other countries, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is the only vaccine recommended by the US Advisory Committee on Immunization Practices (ACIP) and several other countries for all adults 65 years of age and older as well as younger adults with certain chronic conditions [1]. Like PPSV23, the 13-valent pneumococcal conjugate vaccine (PCV13) has been approved by the US Food and Drug Administration and selected other regulators for vaccination of adults aged 50 years and older. The basis for licensure for the adult indication for PCV13 relies on its non-inferior immunogenicity for matched serotypes, when compared to adult responses to PPSV23 [2]. There is no known correlate of protection, or antibody level at which a clinical effect would be expected, for pneumococcal disease in adults, hence it is not possible to translate such immunogenicity measures into expected efficacy or effectiveness measure [1].
In February 2012, the US ACIP chose to continue its 1997 recommendation for routine adult use of PPSV23 rather than recommend routine use of PCV13, until more data become available related to clinical effectiveness of PCV13 and the magnitude of indirect reduction in adult disease due to routine vaccination of children with PCV13 [2, 3]. In June 2012, the ACIP voted to recommend a sequential regimen of PCV13 and PPSV23 for certain high-risk adults with immunocompromising conditions, functional or anatomic asplenia, cerebrospinal leaks, or cochlear implants. These recommendations were published in October 2012 [1].
The ACIP decision-making process included assessments of cost-effectiveness data comparing PCV13 and PPSV23 in adults. Such cost-effectiveness models require explicit assumptions of effectiveness to be specified; however, there are neither any published clinical-outcomes data that compare the two vaccines in adult populations, nor are there any published trials evaluating the efficacy or effectiveness of PCV13 in healthy adults against either invasive pneumococcal disease (IPD) or non-bacteremic pneumococcal pneumonia (NBPP). Several clinical trials and observational studies have evaluated the efficacy and effectiveness of PPSV23 in preventing both invasive and non-invasive diseases [4–6], and there are several meta-analyses of these data [7–9]. The data are consistent regarding PPSV23 effectiveness for invasive disease; however, there are inconsistent meta-analysis results and interpretations regarding PPSV23 effectiveness for preventing non-invasive disease (i.e., NBPP) [7, 8].
Recently published economic analyses described the potential economic impact of different adult pneumococcal vaccine regimens. A paper by Smith et al. [9] modeled the potential economic impact of various vaccination strategies on pneumococcal disease among adults 50 years of age and older. This analysis compared PCV13 to PPSV23, concluding that routine use of PCV13 at ages 50 and 65 would be the most economically efficient recommendation to prevent pneumococcal disease in adults [10]. A separate paper by Weycker et al. [11] yielded similar results. Results from both models were sensitive to effectiveness assumptions for each vaccine, and both models included the assumption that PPSV23 was ineffective in the prevention of NBPP [10, 11]. However, neither of these publications explored the economic implications of the most recent ACIP sequential PCV13–PPSV23 regimen in selected high-risk populations.
Hence, the main objectives of the current analyses are to:
-
1.
evaluate the economic implications of the 2012 ACIP recommendation compared with the 1997 ACIP recommendation;
-
2.
examine additional relevant vaccination strategies that may be considered by decision-makers in the future, and;
-
3.
explore the implications of various assumptions including those pertaining to vaccine effectiveness and herd protection on cost-effectiveness conclusions.
Methods
All appendices mentioned below can be found in the supplementary material published alongside the online version of this article. The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Model Structure
A static cohort model with a 1-year cycle length was developed in Microsoft Excel® (Microsoft Corporation, Albuquerque, NM, USA). The model projects the lifetime risk and associated costs and quality-adjusted life-years (QALY) associated with IPD and NBPP in a hypothetical US 50-year-old adult cohort for a period of 50 years or until death. A 3% discount rate for costs and benefits was used.
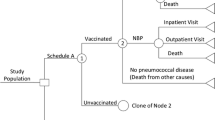
Based on risk and outcome of pneumococcal disease, five health states are modeled (see Appendix 1):
-
1.
Healthy: immunocompetent adults with no comorbid conditions;
-
2.
Immunocompetent adults with comorbidities [i.e., immunocompetent adults diagnosed with certain chronic conditions that moderately increase the risk of pneumococcal disease (e.g., diabetes, heart disease, lung disease)];
-
3.
Immunocompromised: adults at greatly increased risk of pneumococcal disease (immunocompromising conditions include certain cancers, immunosuppression due to chemotherapy, radiation therapy, or bone-marrow transplant, asplenia, human immunodeficiency virus infection, and others);
-
4.
Disabled: disability may occur after IPD or NBPP, and;
-
5.
Death: death may be due to pneumococcal disease or other causes.
Persons in the first three states may develop pneumococcal disease. Disease outcomes include outpatient visits for NBPP, hospitalizations for IPD and NBPP, and death or long-term disability associated with IPD and NBPP. Depending on the outcomes of the diseases, persons can remain at the same state, or may move to other states. Background mortality rates from other causes are incorporated into the model, so the total population at risk of pneumococcal disease is reduced in each simulated year (Fig. 1). Annual age-specific transition probabilities among risk groups were derived from Weycker et al. [12] (see Table S9 in Appendix 1, Distribution of Risk Groups). Vaccination strategies were assumed to reduce the baseline risk of pneumococcal disease.
Vaccination Strategies
Given the need to assess two vaccines, three risk categories, and multiple vaccination age points in this economic analysis, many options were considered for defining a reasonable set of vaccination strategies that are both clinically relevant and practically implementable. For modeling purposes, this analysis is restricted to vaccination strategies for adults 50 years of age and older, for whom the 1997 ACIP recommendation (Table 1) is applicable. Vaccination prior to the age of 50 years is not considered here, nor are adults who may have been vaccinated before the age of 50.
To explore various relevant strategies that could plausibly be implemented in the future, we defined three broad sets of strategies, totaling seven different vaccination strategies, as well as the no-vaccination strategy (which models the hypothetical disease incidence in the absence of any adult pneumococcal immunization; Table 2). For each set of strategies, a logical progression of vaccine recommendations was defined, such that different age and health-based risk categories were considered iteratively. The strategies described below are not the only possible strategies, but rather are considered plausible; for example, a vaccination strategy where PPSV23 is not used at all is not presented because PPSV23 is recommended for adult use by ACIP and multiple other national authorities, and the strategy is weakly dominated by other vaccination strategies included here. These seven strategies were developed based on current ACIP policies and logical extensions of the policies, as well as to include and explore vaccination strategies in recently published adult pneumococcal economic analyses [10, 11].
The first set of strategies describes both the 1997 ACIP recommendation (strategy 1; S1) and the 2012 ACIP change for immunocompromised individuals (strategy 2; S2). The second set illustrates how a vaccination strategy might be constructed if decision-makers were to extend the 2012 ACIP recommendation for a sequential PCV13–PPSV23 regimen to include the immunocompetent with comorbidities group (strategy 3; S3) or both the comorbidities and healthy groups (strategy 4; S4). The age points for vaccination for these strategies appear in Table 2.
The third set consists of three additional strategies that could apply if decision-makers preferred simple recommendations for the majority of individuals (healthy and immunocompetent with comorbidities) as most practical to implement (keeping vaccine choice more consistent and making age at vaccination the main patient characteristic that changes). In this set of strategies, the recommendation for the immunocompromised group is fixed, and different recommendations for the healthy and immunocompetent/comorbidities groups are explored. In strategy 5 (S5), PCV13 at age 65 is first added to the 2012 ACIP immunocompromised recommendation, and the healthy and immunocompetent/comorbidities recommendations remain unchanged. Proceeding to strategy 6 (S6), the current PPSV23 recommendations for the healthy group at 65 and the comorbidities group at 50 and 65 are replaced with PCV13. In strategy 7 (S7), the current healthy and immunocompetent with comorbidities recommendations are replaced with the PCV13–PPSV23 regimen.
Model Inputs
Disease Estimates
Estimates of annual rates of each disease outcome were based on data presented by Weycker et al. [12] (see Table S2 in Appendix 1). The rates of IPD, death and disability from IPD, hospitalized NBPP, death and disability from NBPP, and outpatient visits associated with NBPP were calculated, varying by age and risk of pneumococcal status (see Appendix 1 for description). Baseline rates of disease for the no-vaccination strategy were estimated using methods described previously [13].
Proportions of the population that fall into each risk category were based on published literature (see Table S9 in Appendix 1, Distribution of Risk Groups) [12] and individuals were assumed to move from lower risk categories to higher risk categories probabilistically as they aged in the model. The 50-year-old cohort initially included a population that was 52.3% healthy; 36.5% immunocompetent with comorbidities and 11.2% immunocompromised [12].
Cost Estimates
Medical-care costs related to hospitalizations, outpatient visits, and deaths were included in the model and varied by risk status (see Table S2 in Appendix 1) [12]. Vaccine costs were based on Centers for Disease Control and Prevention reports of private-sector prices as of September 10, 2012 [14]. PPSV23 was assumed to be $57.70 per dose and PCV13 to be $120.95 per dose. Administration cost was assumed to be $15.00 per dose for each product. Indirect costs were not included in this analysis. Costs are expressed in 2012 US dollars using medical Consumer Price Index adjustor [15].
Utility Weights
Utility weights associated with IPD and NBPP inpatient cases, as well as baseline utilities for average- and high-risk populations were based on values described by Smith et al. [9] (see Table S9 in Appendix 1). Utility weights associated with outpatient visits for NBPP were based on estimates related to influenza outpatient visits [10]. Duration of an event is incorporated in QALY calculation.
Vaccine Uptake
Vaccine uptake was assumed to vary depending on age and risk category and was based on contemporary US data regarding PPSV23 (see Table S9 in Appendix 1). Vaccine uptake for both PCV13 and PPSV23 was assumed to be the same for each age/risk category described. For routine age-based recommendations (at age 50 years or age 65 years), vaccine uptake was assumed to be 12.0% for healthy, 33.9% for immunocompetent with comorbidities and immunocompromised populations at age 50 years, and 60.1% for all risk categories at age 65 years.
Vaccine Effectiveness
Due to the absence of data comparing PCV13 to PPSV23 efficacy in adults, assumptions around PCV13 effectiveness against IPD and NBPP as well as PPSV23 effectiveness against NBPP were based on expert opinion using estimates generated from a Delphi panel engaged in 2011 for healthy and immunocompetent with comorbidities groups (Table 3; see Appendix 2 for description of Delphi methods). PPSV23 effectiveness against IPD for the healthy and immunocompetent with comorbidities groups was based on Moberley et al. [7] and Smith et al. [9]. Effectiveness estimates described at the June 2012 ACIP meeting were used for the immunocompromised risk group [16] (Table 3; see Appendix 1 for description of the estimation of vaccine effectiveness). In the base-case analysis, PPSV23 was assumed to wane over a period of 10 years. PCV13 was assumed to be effective in healthy adults, as well as immunocompetent adults with chronic conditions and immunocompromised populations. There are no long-term efficacy data available for PCV13 in adults, so it was assumed to wane over a period of 15 years in this model. All vaccine-waning patterns were assumed to be linear.
Projected vaccine impact on disease was based on the effectiveness estimates described in Table 3, along with vaccine uptake and vaccine serotype coverage. Based on surveillance data from 2008, PPSV23 was assumed for the base-case to cover 75.7% of all IPD and NBPP in adults 50–64 years of age, and 64.7% of disease in adults 65 and older. PCV13 was assumed to cover 52.6% of IPD and NBPP in adults 50–64 years of age and 49.9% of disease in those 65 years of age and older [17].
Sensitivity Analyses
Given the uncertainty around several key model parameters, sensitivity analyses are critical to understanding the potential economic implications of the various vaccination strategies explored. Sensitivity analyses were conducted to examine the impact of varying assumptions related to vaccine effectiveness, impact of herd protection on disease incidence and vaccine serotype coverage. In addition, a set of scenario analyses was conducted to examine the potential cost-effectiveness of broad age-based vaccine recommendations starting at age 50.
Projected Impact of Herd Protection
To be conservative, the impact of herd protection was excluded in the base case. To assess model sensitivity to the impact of herd protection on disease incidence and serotype coverage, additional scenarios were evaluated, anticipating some future point when full reduction in adult disease due indirectly to pediatric PCV13 has been realized. Data related to reductions in IPD observed after routine pediatric use of 7-valent pneumococcal conjugate vaccine (PCV7) were applied to the modeled base-case rates of disease to generate projected disease-incidence rates after routine pediatric use of PCV13 [17]. In that future scenario, IPD and NBPP disease reductions post-PCV13 were assumed to be comparable to those seen for IPD reductions post-PCV7.
There are sufficient surveillance data to indicate that a significant herd impact post-PCV7 introduction has already occurred in the US, however, there is no consensus in the published literature regarding the potential herd impact of use of PCV7 on NBPP in adults [18, 19].
The projected herd-impact parameters varied based on certain serotype characteristics. Disease incidence associated with serotypes contained in PCV7 as well as serotypes 1 and 5 were assumed to remain at current low levels. Serotypes 1 and 5 are believed to behave differently than other serotypes in terms of carriage and transmission, so it is not clear how adult disease related to these two serotypes would be affected by pediatric use of PCV13. The incidence of disease associated with the four other new serotypes in PCV13 was assumed to decrease in a pattern similar to the observed reductions in PCV7 types after routine pediatric vaccination began. All other serotypes (PPSV23 unique types and non-vaccine types) were assumed to increase slightly, similar to increases observed post-PCV7 implementation.
Serotype coverage for each vaccine was assumed to vary and three sets of assumptions were applied to three separate sensitivity analyses:
-
1.
Full herd impact: PPSV23 was assumed to cover 68% of disease in adults aged 50–64 years and PCV13 20%; PPSV23 was assumed to cover 56% of disease in adults older than 65 years, and PCV13 18%;
-
2.
Herd impact was assumed to be 50% of that projected for scenario 1;
-
3.
Herd impact for IPD described as in scenario 1, but no herd impact assumed for NBPP. In this scenario NBPP rates of disease and serotype distributions were equivalent to base-case assumptions.
Waning of Effectiveness
Recent studies demonstrate that PPSV23 induces antibody responses that remain above the levels of unvaccinated adults for 5–10 years or more [20]. Little long-term effectiveness data are available for PCV13 in adults. To understand the impact of waning assumptions of cost-effectiveness results, three sensitivity analyses were generated:
-
1.
PCV13 and PPSV23 were assumed to have equivalent waning rates of 10 or 15 years;
-
2.
PCV13 was assumed to wane over a period of 20 years, rather than 15 years as in the base case.
Results
Projected Pneumococcal Disease Events
Total projected IPD cases (survived), NBPP hospitalizations (survived), disabled cases (survived), outpatient visits, and deaths for the no-vaccination strategy, along with the projected events averted for the seven vaccination strategies were generated (Table 4). The model projects that for a single cohort of 100,000 adults starting at age 50 years, in the absence of pneumococcal vaccination, there would be 870 IPD cases, 12,445 NBPP outpatient visits, 7,689 NBPP hospitalizations, and 340 disabled cases due to IPD/NBPP. Total projected events averted for each of the vaccination strategies indicate that strategy 7 would prevent the most events compared to all other strategies (Table 4).
Economic Evaluation
For objective 1, three strategies were compared: no vaccination, as well as S1 (1997 ACIP recommendations) and S2 (2012 ACIP recommendations). In this first comparison, S2 is the most economically efficient strategy with an incremental cost-effectiveness ratio (ICER) of $25,841 per QALY gained, compared with no vaccination, and S1 is dominated.
For objective 2, four possible future vaccination strategies were added to the comparison (Table 5). The most economically efficient strategy is S5, with an ICER of $23,416 per QALY gained compared to no vaccination. S6 had an ICER of $124,665 per QALY gained compared with S5. S7 had an ICER of $182,067 per QALY gained compared to S6. Other strategies are either dominated or less efficient.
Sensitivity Analysis Results
Sensitivity analyses related to impact of disease incidence and serotype changes and due to herd protection along with changes to vaccine effectiveness assumptions are reported in Table 6.
Herd Protection
The potential impact on cost-effectiveness results due to projected changes in disease incidence for both IPD and NBPP along with serotype coverage for each vaccine was examined using three sensitivity analysis scenarios. For the first scenario, simulating the impact of full herd protection, the three most economically efficient strategies were S1 ($51,000 vs. no vaccination), followed by S2 ($77,000 vs. S1) and S5 ($103,000 vs. S2). If 50% less herd impact were to occur, S5 would be the most economically efficient ($36,000 vs. no vaccination), followed by S7. When it was assumed that herd protection did not impact incidence of NBPP, S1 was found to be the most economically efficient strategy followed by S5 and S3 (Table 6).
PPSV23 Effectiveness Against NBPP
When PPSV23 vaccine effectiveness for preventing NBPP was assumed to be 10% greater than base case in the first year post-vaccination, the order of preferred vaccination strategies in the economic analysis were maintained but with ICERs reduced.
Reductions in PPSV23 effectiveness (from 10% to 100% less relative to first year post-vaccination in the base case) did not affect S5 to be the most economically efficient strategy. But the economic efficiency for S6 improved substantially, with ICER reducing from $150,000 to $39,000 against S5. When PPSV23 effectiveness against NBPP was decreased by 100% (i.e., assumed to be 0) versus base case, S5 and S6 became similarly efficient (ICER $35,000 and $36,000, respectively, per QALY gained vs. no vaccination).
Waning of Effectiveness
Vaccine-waning patterns were found to impact cost-effectiveness results in the sensitivity analyses. When the two vaccines were assumed to have equivalent waning periods of 10 years, the most economically efficient strategies were S1 followed by S2 and S5, and all had ICERs below $50,000 per QALY.
Discussion
The results of this analysis are intended to provide insight into the cost-effectiveness of alternative pneumococcal vaccination policies for adults in the United States. Although there is no consensus on a threshold for good value for resources expended, we present our results in the context of commonly cited thresholds of incremental cost per QALY of $ 50,000 or $100,000 [21]. The base-case results indicate that the 2012 ACIP recommendations for adults with certain immunocompromising and other conditions that place them at highest risk for pneumococcal disease would be considered cost-effective when compared to the 1997 ACIP recommendations. When exploring future potential vaccination strategies, we found the most efficient strategy to be similar to the 2012 ACIP recommendations, with the addition of another dose of PCV13 for the immunocompromised population at age 65, followed by the usual dose of PPSV23.
Several limitations related to availability of data apply to this analysis. No trials with clinical disease endpoints comparing these two vaccines are available. There are no clinical efficacy data available for PCV13 in healthy adults, nor are there data available related to the duration of PCV13 effectiveness in adults. Diagnosis of NBPP is challenging, as most pneumonia episodes are evaluated and treated empirically, without determination of etiology. Disease-incidence estimates were generated based on a published analysis of hospitalization databases [12]. There were no data available related to serotype distribution for NBPP, so serotype distributions for NBPP were assumed to be the same as for IPD. Several limitations relate to assumptions used in sensitivity analysis around impact of herd protection. Two older publications reported conflicting findings: an analysis of claims-based data suggests that there has been no significant impact of PCV7 on adult NBPP [18]; however, another study, utilizing different methods and incorporating techniques to adjust for seasonality and to address limitations in data sources, reported significant declines in NBPP in adults post-PCV7 introduction [19]. A more recent study estimated that there has been a substantial reduction in adult pneumonia hospitalizations during the post-PCV7 vaccine introduction years (2007–2009) compared with the pre-PCV7 years (1997–1999) [22]. Additionally, our model is based on a US 50-year-old cohort and does not consider cohorts of differing ages.
When our sensitivity analyses accounted for the herd effects of pediatric use of PCV13 on adult disease, we found that vaccination strategies including PCV13 may not be efficient if adult disease due to the 13 serotypes is reduced more than 50% from pre-PCV13 levels. In that scenario, we found the most efficient pneumococcal vaccination policy to be the 1997 ACIP recommendation. Our accounting for the herd effects of PCV13 may differ from previous cost-effectiveness analyses, insofar as neither Smith et al. [9] nor Weycker et al. [11] assumed that routine pediatric use of PCV13 would have a substantial impact on NBPP in adults. Consistent evidence to support this assumption is lacking. However, the recent study by Griffin et al. [22] lends support for our sensitivity analysis assumptions related to the herd impact on NBPP in adults. Future studies of pneumococcal pneumonia incidence data should attempt to determine the impact of the pediatric use of PCV13 on non-IPD in adults.
These results differ significantly from previous analyses, due, in part, to our PPSV23 NBPP effectiveness assumptions, which were obtained through a Delphi panel process. For example, Smith et al. [9] also used a Delphi panel to estimate PCV13 effectiveness against NBPP; however, they did not report Delphi estimates for PPSV23 effectiveness against NBPP. Instead, the authors assumed the effectiveness of PPSV23 was zero in the base-case analysis [10]. Sensitivity analyses conducted as part of the Smith et al. [9] study indicated that if PPSV23 was assumed to be effective in the prevention of NBPP, then a broad recommendation for the use of PPSV23 would be the most efficient vaccination strategy in their analyses. Their sensitivity analysis result is consistent with our base-case findings. Even when PPSV23 is assumed to be ineffective against NBPP, our sensitivity analysis showed that S5 (adding PCV13 to immunocompromised group only) is still the most efficient although the efficiency of S6 (substituting PCV13 for PPSV13 for healthy and immunocompetent in addition to adding PCV13 to immunocompromised group) approached S5. However, it is expected that S6 will have a significantly larger budget impact given the cost of PCV13. Another economic analysis recently published by Weycker et al. [11] also reported results that favored broad PCV13 immunization. The Weycker et al. [11] analysis examined the impact of pneumococcal vaccines on all-cause pneumonia, rather than restricting the analysis to NBPP as we have done. Weycker et al. [11] used effectiveness assumptions similar to those used in Smith et al. [9] for PPSV23, and they used pediatric data for pneumonia effectiveness assumptions for adult use of PCV13. Weycker et al. [11] did not explore whether their conclusions were influenced by their assumptions regarding the effectiveness of PPSV23 in preventing NBPP. Furthermore, most strategies considered by Weycker et al. [11] assumed use of PCV13 in all adults age ≥50 years at model entry, which were inconsistent with ACIP recommendations. We evaluated strategies with vaccination at age 50 for the lower risk population (details not reported) and found that these strategies were less economically efficient than the seven strategies explored in the analysis. As a result, we cannot compare our findings with their results.
Conclusion
The 2012 ACIP recommendation is the more economically efficient strategy comparing to the 1997 ACIP recommendation. However, modifying the 2012 recommendation to include an additional dose of PCV13 at age 65, followed by PPSV23, for adults with immunocompromising conditions appears to be a more cost-effective vaccine policy than the 2012 ACIP recommendation.
The results of our analyses demonstrate the influence of parameters such as vaccine effectiveness and herd protection effects on economic evaluations of adult pneumococcal vaccine policies. PPSV23 protects against infection with 23 serotypes and prevents IPD, the most serious manifestation of this infection. Given that the herd protection effects of routine pediatric PCV13 use are likely to reduce the utility of PCV13 in adults, the additional protection conferred by PPSV23 will be important for preventing IPD due to the serotypes not covered in PCV13. Due to the uncertainty in available data, as well as the absence of key influential data, comprehensive sensitivity analyses should be conducted by policy-makers when evaluating new pneumococcal vaccine strategies in adults.
References
Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816–9.
Centers for Disease Control and Prevention. Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012;61:394–5.
Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46:1–24.
Fedson D, Nicolas-Spony L, Klemets P, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011;10:1143–67.
Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
Fedson DS, Liss C. Precise answers to the wrong question: prospective clinical trials and the meta-analyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine. 2004;22:927–46.
Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008;CD000422.
Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58.
Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307:804–12. http://www.ncbi.nlm.nih.gov/pubmed/22357831.
Siddiqui MR, Edmunds WJ. Cost-effectiveness of antiviral stockpiling and near-patient testing for potential influenza pandemic. Emerg Infect Dis 2008;14:267–74.
Weycker D, Sato R, Strutton D, Edelsberg J, Atwood M, Jackson LA. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥50 years. Vaccine. 2012;30:5437–44.
Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28:4955–60.
Fry AM, Zell ER, Schuchat A, Butler JC, Whitney CG. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine. 2002;21:303–11.
Centers for Disease Control and Prevention. Vaccines for Children Program (VFC). Archived CDC vaccine price list as of September 10, 2012. http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/2012/2012-09-10.html. Last accessed October 6, 2013.
Bureau of Labor Statistics, United States Department of Labor, Crawford M, Church J, Rippy D. (eds.). CPI detailed report data for December 2012. http://www.bls.gov/cpi/cpid1212.pdf. Last accessed March 17, 2014.
Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices, summary report, June 20–21. Atlanta: Centers for Disease Control and Prevention; 2012. http://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-jun12.pdf. Last accessed October 6, 2013.
Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41.
Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86.
Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2:e00309–10.
Grabenstein JD, Manoff SB. Pneumococcal polysaccharide 23-valent vaccine: long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine. 2012;30:4435–44.
Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28.
Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. US hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63.
Acknowledgments
Funding was provided from Merck for publication of this article. We gratefully acknowledge the following individuals for their contributions to the analysis and preparation of this manuscript: Karin Travers, Rebecca Sugarman, and Thomas Weiss for development of the Delphi protocol and execution of that project; Y. Adam Cui for assistance with model inputs and interpretation; Minfu He for programming assistance; Kyung Min Song for editing assistance; Leona Markson for her guidance throughout the project and thoughtful review and comment on early drafts of the manuscript. No medical writing assistance was received during the preparation of this manuscript. All authors meet the ICMJE criteria for authorship for this manuscript and have made substantial contributions to the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Conflict of interest
Jieling Chen is an employee of Merck Sharp & Dohme Corporation, and may own stock and/or stock options in the company. Megan A. O’Brien is an employee of Merck & Co., Inc., and may own stock and/or stock options in the company. H. Keri Yang is an employee of Merck & Co., Inc., and may own stock and/or stock options in the company. John D. Grabenstein is an employee of Merck & Co., Inc., and may own stock and/or stock options in the company. Erik J. Dasbach is an employee of Merck & Co., Inc., and may own stock and/or stock options in the company. Merck Sharp & Dohme Corporation, a subsidiary of Merck & Co., Inc., is the manufacturer of 23-valent pneumococcal polysaccharide vaccine and developer of a candidate 15-valent pneumococcal conjugate vaccine.
Compliance with ethics guidelines
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, J., O’Brien, M.A., Yang, H.K. et al. Cost-Effectiveness of Pneumococcal Vaccines for Adults in the United States. Adv Ther 31, 392–409 (2014). https://doi.org/10.1007/s12325-014-0115-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0115-y