Abstract
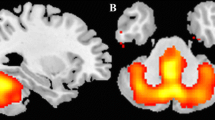
Cognitive and olfactory impairments have previously been demonstrated in patients with spinocerebellar ataxia type 3 (SCA3), also known as Machado–Joseph disease (MJD)—SCA3/MJD. We investigated changes in regional cerebral blood flow (rCBF) using single-photon emission computed tomography (SPECT) imaging in a cohort of Brazilian patients with SCA3/MJD. The aim of the present study was to evaluate the correlation among rCBF, cognitive deficits, and olfactory dysfunction in SCA3/MJD. Twenty-nine genetically confirmed SCA3/MJD patients and 25 control subjects were enrolled in the study. The severity of cerebellar symptoms was measured using the International Cooperative Ataxia Rating Scale and the Scale for the Assessment and Rating of Ataxia. Psychiatric symptoms were evaluated by the Hamilton Anxiety Scale and Beck Depression Inventory. The neuropsychological assessment consisted of Spatial Span, Symbol Search, Picture Completion, the Stroop Color Word Test, Trail Making Test (TMT), and Phonemic Verbal Fluency. Subjects were also submitted to odor identification evaluation using the 16-item Sniffin’ Sticks. SPECT was performed using ethyl cysteine dimer labeled with technetium-99m. SCA3/MJD patients showed reduced brain perfusion in the cerebellum, temporal, limbic, and occipital lobes compared to control subjects (pFDR <0.001). A significant positive correlation was found between the Picture Completion test and perfusion of the left parahippocampal gyrus and basal ganglia in the patient group as well as a negative correlation between the TMT part A and bilateral thalamus perfusion. The visuospatial system is affected in patients with SCA3/MJD and may be responsible for the cognitive deficits seen in this disease.

Similar content being viewed by others
References
Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100:443–54.
Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–87.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44.
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304.
Lima L, Coutinho P. Clinical criteria for diagnosis of Machado-Joseph disease: report of a non-Azorean Portuguese family. Neurology. 1980;30:319–22.
Jardim LB, Pereira ML, Silveira I, Ferro A, Sequeiros J, Giugliani R. Neurologic findings in Machado-Joseph disease: relation with disease duration, subtypes, and (CAG)n. Arch Neurol. 2001;58:899–904.
Braga-Neto P, Felicio AC, Pedroso JL, Dutra LA, Bertolucci PHF, Gabbai AA, et al. Clinical correlates of olfactory dysfunction in spinocerebellar ataxia type 3. Parkinsonism Relat Disord. 2011;17:353–6.
Pedroso JL, Braga-Neto P, Felício AC, Dutra LA, Santos WAC, Do Prado GF, et al. Sleep disorders in Machado–Joseph disease: frequency, discriminative thresholds, predictive values and correlation with ataxia-related motor and non-motor features. Cerebellum. 2011;10:291–5.
Radvany J, Camargo CHP, Costa ZM, Fonseca NC, Nascimento ED. Machado Joseph disease of Azorean ancestry in Brazil: the Catarina kindred. Neurological, neuroimaging, psychiatric and neuropsychological findings in the largest known family, the Catarina kindred. Arq Neuropsiquiatr. 1993;51:21–30.
Bürk K, Globas C, Bösch S, Klockgether T, Zühlke C, Daum I, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250:207–11.
Klinke I, Minnerop M, Schmitz-Hübsch T, Hendriks M, Klockgether T, Wüllner U, et al. Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum. 2010;9:433–42.
Zawacki TM, Grace J, Friedman JH, Sudarsky L. Executive and emotional dysfunction in Machado-Joseph disease. Mov Disord. 2002;17:1004–10.
Maruff P, Tyler P, Burt T, Currie B, Burns C, Currie J. Cognitive deficits in Machado-Joseph disease. Ann Neurol. 1996;40:421–7.
Kawai Y, Takeda A, Abe Y, Washimi Y, Tanaka F, Sobue G. Cognitive impairments in Machado-Joseph disease. Arch Neurol. 2004;61:1757–60.
Braga-Neto P, Pedroso JL, Alessi H, Dutra LA, Felício AC, Minett T, et al. (2012) Cerebellar cognitive affective syndrome in Machado Joseph disease: core clinical features. Cerebellum. doi:10.1007/s12311-011-0318-6
Manto M, Lorivel T. Cognitive repercussions of hereditary cerebellar disorders. Cortex. 2011;47:81–10.
Abele M, Riet A, Hummel T, Klockgether T, Wüllner U. Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J Neurol. 2003;250:1453–5.
Connelly T, Farmer JM, Lynch DR, Doty RL. Olfactory dysfunction in degenerative ataxias. J Neurol Neurosurg Psychiatr. 2003;74:1435–7.
Velázquez-Pérez L, Fernandez-Ruiz J, Díaz R, González RP, Ochoa NC, Cruz GS, et al. Spinocerebellar ataxia type 2 olfactory impairment shows a pattern similar to other major neurodegenerative diseases. J Neurol. 2006;253:1165–9.
Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18(4):364–72.
Takahashi N, Odano I, Nishihara M, Yuasa T, Sakai K. Regional cerebral blood flow measured with N-isopropyl-p-[123I]iodoamphetamine single-photon emission tomography in patients with Joseph disease. Eur J Nucl Med. 1994;21:615–20.
Etchebehere EC, Cendes F, Lopes-Cendes I, Pereira JA, Lima MC, Sansana CR, et al. Brain single-photon emission computed tomography and magnetic resonance imaging in Machado-Joseph disease. Arch Neurol. 2001;58:1257–63.
Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome: the Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11.
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20.
Braga-Neto P, Godeiro-Junior C, Dutra LA, Pedroso JL, Barsottini OG. Translation and validation into Brazilian version of the Scale of the Assessment and Rating of Ataxia (SARA). Arq Neuropsiquiatr. 2010;68:228–30.
Wechsler D. Wechsler memory scale–revised manual. San Antonio: Psychological Corporation; 1987.
Wechsler D. Wechsler adult intelligence scale, 3rd edition (WAIS III): test manual. 3rd ed. New York: The Psychological Corporation; 1997.
Weschsler D. WAIS-III: Escala de inteligência para adultos: Manual para administração e avaliação. 1st ed. São Paulo: Casa do Psicólogo; 2004.
Spreen O, Strauss E. A compendium of neuropsychological tests. 2nd ed. New York: Oxford University Press; 1998.
Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Motor Skill. 1958;8:271–6.
Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 2004.
Silveira-Moriyama L, Carvalho Mde J, Katzenschlager R, Petrie A, Ranvaud R, Barbosa ER, et al. The use of smell identification tests in diagnosis of Parkinson’s disease in Brazil. Mov Disord. 2008;23:2328–34.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Rüb U, Brunt ER, Deller T. New insights into the pathoanatomy of spinocerebellar ataxia type 3 (Machado Joseph disease). Curr Opin Neurol. 2008;21:111–6.
Paulson HL, Das SS, Crino PB, Perez MK, Patel SC, Gotsdiner D, et al. Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain. Ann Neurol. 1997;41:453–62.
Yamada M, Hayashi S, Tsuji S, Takahashi H. Involvement of the cerebral cortex and autonomic ganglia in Machado-Joseph disease. Acta Neuropathol. 2001;101:140–4.
D'Abreu A, França Jr MC, Yasuda CL, Campos BA, Lopes-Cendes I, Cendes F. Neocortical atrophy in Machado-Joseph disease: a longitudinal neuroimaging study. J Neuroimaging. 2012. doi:10.1111/j.1552-6569.2011.00614.x
Leh SE, Petrides M, Strafella AP. The neural circuitry of executive functions in healthy subjects and Parkinson's disease. Neuropsychopharmacology. 2010;35:70–85.
Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–30.
Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. 2010;16:466–87.
Doeller CF, Kaplan R. Parahippocampal cortex: translating vision into space. Curr Biol. 2011;21(15):R589–91.
Macevoy SP, Epstein RA. Constructing scenes from objects in human occipitotemporal cortex. Nat Neurosci. 2011;14:1323–9.
Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus and cerebrocerebellar systems. Cortex. 2008;44:1037–66.
Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200.
Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–70.
Rüb U, de Vos RA, Brunt ER, Sebestény T, Schöls L, Auburger G, et al. Spinocerebellar ataxia type 3 (SCA3): thalamic neurodegeneration occurs independently from thalamic ataxin-3 immunopositive neuronal intranuclear inclusions. Brain Pathol. 2006;16:218–27.
De Oliveira MS, D'Abreu A, França Jr MC, Lopes-Cendes I, Cendes F, Castellano G. MRI-Texture analysis of corpus callosum, thalamus, putamen, and caudate in Machado-Joseph disease. J Neuroimaging. 2012. doi:10.1111/j.1552-6569.2010.00553.x
Crowe SF. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J Clin Psychol. 1998;54:585–91.
Tanaka M, Kunimatsu J. Contribution of the central thalamus to the generation of volitional saccades. Eur J Neurosci. 2011;33:2046–57.
Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;28(71):209–23.
Molinari M, Leggio MG. Cerebellar information processing and visuospatial functions. Cerebellum. 2007;6:214–20.
Meyer JS, Obara K, Muramatsu K. Diaschisis. Neurol Res. 1993;15:362–6.
Kim YT, Shin SM, Lee WY, Kim GM, Jin DK. Expression of expanded polyglutamine protein induces behavioral changes in Drosophila (polyglutamine-induced changes in Drosophila). Cell Mol Neurobiol. 2004;24:109–22.
Goti D, Katzen SM, Mez J, Kurtis N, Kiluk J, Ben-Haïem L, et al. A mutant ataxin-3 putative-cleavage fragment in brains of Machado-Joseph disease patients and transgenic mice is cytotoxic above a critical concentration. J Neurosci. 2004;24:10266–79.
Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18(21):8990–9001.
Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, Inoue K, et al. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 2000;84:1656–66.
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). We would like to thank the patients, their families, and the health volunteers for participating in this study.
Conflict of interest
Information concerning all sources of financial support and funding for the preceding 12 months is disclosed below, regardless of relationship to the current manuscript, submitted under the following suggested categories. Rodrigo A. Bressan was a consultant and a member of the advisory board of AstraZeneca and Janssen-Cilag; had partnerships with FAPESP, CNPq, and Instituto Albert Einstein de Ensino e Pesquisa; received honoraria from Eli Lilly, AstraZeneca, Janssen-Cilag, and Novartis; and received grants from FAPESP, CNPq, and Instituto Albert Einstein de Ensino e Pesquisa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braga-Neto, P., Dutra, L.A., Pedroso, J.L. et al. Cognitive Deficits in Machado–Joseph Disease Correlate with Hypoperfusion of Visual System Areas. Cerebellum 11, 1037–1044 (2012). https://doi.org/10.1007/s12311-012-0354-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0354-x