Abstract
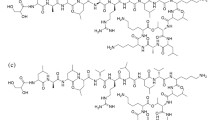
KRK-701P is a broad-spectrum antibiotic with methicillin-resistant antibacterial activity. The objective of this preformulation study was to determine the physicochemical properties of KRK-701P. The n-octanol-to-water partition coefficients of KRK-701P were 0.448 at pH 4 and 2.546 at pH 8. One crystal form and one amorphous form of KRK-701P were isolated by recrystallization and characterized by differential scanning calorimetry, powder X-ray diffractometry and thermogravimetric analysis. After storage for 1 month at 0 % RH (silica gel, 20 °C), 52 % RH (saturated solution of Na2Cr2O7.2H2O/20 °C) and 95 % RH (saturated solution of Na2HPO4/20 °C), the two forms were not transformed.






Similar content being viewed by others
References
Aulton, M. E., 2002. The science of dosage form design. Pharmaceutics, second edition. Churchill Livingstone, Edinburgh pp. 113–138.
Bachet, B. 1997. X-ray characterization of the triclinic polymorph of carbamazepine. Journal of Pharmaceutical Sciences 86: 1062–1065.
Campeta, A.M., B.P. Chekal, Y.A. Abramov, P.A. Meenan, M.J. Henson, B. Shi, R.A. Singer, and K.R. Horspool. 2010. Development of a targeted polymorph screening approach for a complex polymorphic and highly solvating API. Journal of Pharmaceutical Sciences 99: 3874–3886.
Cho, H.J., P. Balakrishnan, H. Lin, M.K. Choi, and D.D. Kim. 2012. Application of biopharmaceutics classification system (BCS) in drug transport studies across human respiratory epithelial cell monolayers. Journal of Pharmaceutical Investigation 42: 147–153.
Craig, D.Q.M., P.G. Rotall, V.L. Kett, and M.L. Hopton. 1999. The relevance of the amorphous state to pharmaceutical dosage forms: Glassy drugs and freeze dried systems. International Journal of Pharmaceutics 179: 179–207.
Do, E.S., and Y.T. Sohn. 2011. Solid state of tulobuterol: Characterization, dissolution, transformation. Journal of Pharmaceutical Investigation 41: 371–376.
Fiese, E. F. and Hagen, T. A., Preformulation. In: Lachman L, Lieberman HA, Kanig JL 1986. (eds) The theory and practice of industrial pharmacy, Lea & Febiger, Philadelphia, pp 171-185.
Giron, D. 1995. Thermal analysis and calorimetric methods in the characterization of polymorphs and solvates. Thermochimica Acta 248: 1–59.
Guillory, J. K. 1999. Generation of polymorphs, hydrates, solvates, and amorphous solids. In Brittain, HG. ed Polymorphism in pharmaceutical solids. Drugs and the pharmaceutical sciences Vol. 95. Marcel Dekker, .Basel, pp 208–209.
Haleblian, J.K., and W.C. McCrone. 1969. Pharmaceutical applications of polymorphism. Journal of Pharmaceutical Sciences 58: 911–929.
Haleblian, J.K. 1975. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. Journal of Pharmaceutical Sciences 64: 1269–1288.
Han, Z., Y. Zheng, N. Chen, L. Luan, C. Zhou, L. Gan, and Y. Wu. 2008. Simultaneous determination of four alkaloids in Lindera aggregate by ultra-high-pressure liquid chromatography-tandem mass spectrometry. Journal of Chromatography A 1212: 76–81.
Hancock, B.C., and G. Zografi. 1997. Characteristics and significance of the amorphous state in pharmaceutical systems. Journal of Pharmaceutical Sciences 86: 1–12.
Hilden, L.R., and K.R. Morris. 2004. Physics of amorphous solids. Journal of Pharmaceutical Sciences 93: 3–12.
Kim, B. J., Kim, J. H., Lee, E. T., Heor, J. H. 2009. The new production method of 2-arylmethyl azetidine carbapenem-3-carboxylate ester. WO 2009/066917.
Lee, E.A., and Y.T. Sohn. 2008. Crystal forms of a capsaicin derivative analgesic DA-5018. Journal of Thermal Analysis and Calorimetry 93: 871–874.
Oguchi, T., N. Sasaki, T. Hara, Y. Tozuka, and K. Yamamoto. 2003. Differential thermal crystallization from amorphous chenodeoxycholic acid between the ground specimens derived from the polymorphs. International Journal of Pharmaceutics 253: 81–88.
Opalchenova, G., and G.N. Kalinkova. 1997. Evaluation of a new polymorph azlocillin sodium by its antibacterial activity. International Journal of Pharmaceutics 153: 263–265.
Price, R., and P.M. Young. 2004. Visualization of the crystallization of lactose from the amorphous state. Journal of Pharmaceutical Sciences 93: 155–164.
Pyne, A., K. Chatterjee, and R. Suryanarayanan. 2003. Crystalline to amorphous transition of disodium hydrogen phosphate during primary drying. Pharmaceutical Research 20: 802–803.
Rivera, A.B., and R.G. Hernandez. 2002. Physico-chemical and solid-state characterization of secnidazole. Il Farmaco 55: 700.
Sohn, Y.T. 2004. Effect of crystal form on bioavailability. J. Pharm. Invest. 34: 443–452.
Sohn, Y.T. 2007. Crystal forms of an angiotensin II receptor antagonist BR-A657. Jounal of Thermal Analysis and Calorimetry 89: 799–802.
Song, H.O., and Y.T. Sohn. 2010. Crystal forms of SK-3530. Archives of Pharmacal Research 12: 2033–2036.
Tang, X.C., M.J. Pikal, and L.S. Taylor. 2002. The effect of temperature on hydrogen bonding in crystalline and amorphous phases in dihydropyrine calcium channel blockers. Pharmaceutical Research 19: 484–490.
Telang, C., L. Yu, and R. Suryanarayanan. 2003. Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride. Pharmaceutical Research 20: 660–667.
Yamamura, S., and Y. Momose. 2001. Quantitative analysis of crystalline pharmaceuticals in powders and tablets by a pattern-fitting procedure using X-ray powder diffraction data. International Journal of Pharmaceutics 212: 203–212.
Yu, L. 2001. Amorphous pharmaceutical solids. Preparation, characterization and stabilization. Advanced Drug Delivery Reviews 48: 27–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bang, I.J., Kim, B.J. & Sohn, Y.T. Characterization of the physicochemical properties of KRK-701P. Arch. Pharm. Res. 38, 834–838 (2015). https://doi.org/10.1007/s12272-014-0450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0450-1