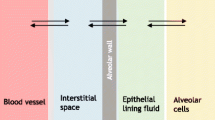
Abstract
Transport studies of model drugs were conducted across the human nasal epithelial (HNE) and normal human bronchial epithelial (NHBE) cell monolayers cultured by air–liquid interface method. Physicochemical properties (e.g., molecular weight, calculated partition coefficient, dose number) of model drugs were quoted from literatures and apparent permeability coefficients (P app) across the HNE and NHBE cell monolayers were directly measured. A linear relationship was observed between the P app values of model drugs in the HNE and NHBE cell monolayers. As the molecular weight of model drugs increased, the P app showed a decreasing pattern while the increase of partition coefficients resulted in the increment of P app. These results indicated that the transport of model drugs across both cell monolayers followed mainly the passive diffusion mechanism, although substrates mediated by drug transporters showed a deviating pattern. It was also interesting to note that almost all model drugs could be grouped into the same biopharmaceutics classification system as that classified by the human intestinal permeability when the P app was plotted as a function of dose number (D 0) of each drug.




Similar content being viewed by others
References
Abe K, Irie T, Uekama K (1995) Enhanced nasal delivery of luteinizing hormone releasing hormone agonist buserelin by oleic acid solubilized and stabilized in hydroxypropyl-beta-cyclodextrin. Chem Pharm Bull 43:2232–2237
Bai S, Yang T, Abbruscato TJ, Ahsan F (2008) Evaluation of human nasal RPMI 2650 cells grown at an air-liquid interface as a model for nasal drug transport studies. J Pharm Sci 97:1165–1178
Cho HJ, Choi MK, Lin H, Kim JS, Chung SJ, Shim CK, Kim DD (2011) Expression and functional activity of P-glycoprotein in passaged primary human nasal epithelial cell monolayers cultured by the air-liquid interface method for nasal drug transport study. J Pharm Pharmacol 63:385–391
Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC (2007) Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm 337:1–24
Densmore CL, Orson FM, Xu B, Kinsey BM, Waldrep JC, Hua P, Bhogal B, Knight V (2000) Aerosol delivery of robust polyethyleneimine-DNA complexes for gene therapy and genetic immunization. Mol Ther 1:180–188
Forbes B, Shah A, Martin GP, Lansley AB (2003) The human bronchial epithelial cell line 16HBE14o: as a model system of the airways for studying drug transport. Int J Pharm 257:161–167
Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM (2003) Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 311:31–45
Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B (2006) Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res 23:1482–1490
Hermens WA, Deurloo MJ, Romeyn SG, Verhoef JC, Merkus FW (1990) Nasal absorption enhancement of 17 beta-estradiol by dimethyl-beta-cyclodextrin in rabbits and rats. Pharm Res 7:500–503
Hinchcliffe M, Illum L (1999) Intranasal insulin delivery and therapy. Adv Drug Deliv Rev 35:199–234
Horvath G, Schmid N, Fragoso MA, Schmid A, Conner GE, Salathe M, Wanner A (2007) Epithelial organic cation transporters ensure pH-dependent drug absorption in the airway. Am J Respir Cell Mol Biol 36:53–60
Kaler G, Truong DM, Sweeney DE, Logan DW, Nagle M, Wu W, Eraly SA, Nigam SK (2006) Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem Biophys Res Commun 351:872–876
Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernäs H, Hussain AS, Junginger HE, Stavchansky SA, Midha KK, Shah VP, Amidon GL (2004) Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol Pharm 1:85–96
Kim JS, Mitchell S, Kijek P, Tsume Y, Hilfinger J, Amidon GL (2006) The suitability of an in situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests. Mol Pharm 3:686–694
Laursen T, Grandjean B, Jorgensen JO, Christiansen JS (1996) Bioavailability and bioactivity of three different doses of nasal growth hormone (GH) administered to GH-deficient patients: comparison with intravenous and subcutaneous administration. Eur J Endocrinol 135:309–315
Lee MK, Yoo JW, Lin H, Kim YS, Kim DD, Choi YM, Park SK, Lee CH, Roh HJ (2005) Air-liquid interface culture of serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Drug Deliv 12:305–311
Lin H, Lee CH, Shim CK, Chung SJ, Kim DD (2004) In vitro transport of fexofenadine · HCl in deformable liposomes across the human nasal epithelial cell monolayers. J Kor Pharm Sci 34:483–489
Lin H, You JW, Roh HJ, Lee MK, Chung SJ, Shim CK, Kim DD (2005) Transport of anti-allergic drugs across the passage cultured human nasal epithelial cell monolayer. Eur J Pharm Sci 26:203–210
Lin H, Li H, Cho HJ, Bian S, Roh HJ, Lee MK, Kim JS, Chung SJ, Shim CK, Kim DD (2007) Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J Pharm Sci 96:341–350
Lu D, Hickey AJ (2007) Pulmonary vaccine delivery. Expert Rev Vaccines 6:213–226
Paruta AN, Sciarrone BJ, Lordi NG (1965) Solubility profiles for the xanthines in dioxane-water mixtures. J Pharm Sci 54:838–841
Ross DL, Riley CM (1990) Aqueous solubilities of variously substituted quinolone antimicrobials. Int J Pharm 63:237–250
Wang Z, Zhang Q (2004) Transport of proteins and peptides across human cultured alveolar A549 cell monolayer. Int J Pharm 269:451–456
Wang X, He H, Leng W, Tang X (2006) Evaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis method. Int J Pharm 317:40–46
Wioland MA, Fleury-Feith J, Corlieu P, Commo F, Monceaux G, Lacau-St-Guily J, Bernaudin JF (2000) CFTR, MDR1, and MRP1 immunolocalization in normal human nasal respiratory mucosa. J Histochem Cytochem 48:1215–1222
You JW, Kim YS, Lee SH, Lee MK, Roh HJ, Jhun BH, Lee CH, Kim DD (2003) Serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Pharm Res 20:1690–1696
Acknowledgments
This work was supported by the Industrial Source Technology Development Program funded by the Ministry of Commerce, Industry and Energy (MOCIE) (No. 10031825) and by the MarineBio Research Program (NRF-C1ABA001-2011-0018561) of the National Research Foundation (NRF) grant funded by the Korean government (MEST).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, HJ., Balakrishnan, P., Lin, H. et al. Application of biopharmaceutics classification system (BCS) in drug transport studies across human respiratory epithelial cell monolayers. Journal of Pharmaceutical Investigation 42, 147–153 (2012). https://doi.org/10.1007/s40005-012-0020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-012-0020-9