Abstract
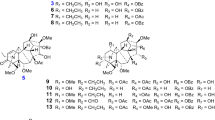
Three novel alkaloids (1–3), together with nineteen known ones (4–22), were isolated from the bulbs of Lycoris longituba. Their structures were elucidated on the basis of extensive spectroscopic analyses, which belong to several Amaryllidaceae alkaloid skeletons. Among them, the harmane-type alkaloids (the new compound 1 and the known compounds 5, 6 and 7) were found for the first time from Lycoris genus. The isolates were tested for their neuroprotective activities against CoCl2, H2O2 and Aβ25–35-induced SH-SY5Y cell injuries, and the majority of them exhibited neuroprotective activities of different degrees. The acetylcholinesterase (AChE) inhibitory activities of the isolated alkaloids were also evaluated, while compounds 12, 14–20 and 22 exhibited extremely significant AChE inhibitory activities.




Similar content being viewed by others
References
Abramovitch, R.A., and I.D. Spenser. 1964. The nuclear magnetic resonance spectra of the carbolines. Canadian Journal of Chemistry 42: 954–956.
Alonso, R., A. Caballero, P.J. Campos, and M.A. Rodr I Guez. 2010. Photochemistry of acyloximes: Synthesis of heterocycles and natural products. Tetrahedron 66: 8828–8831.
Andrade, J.P., S. Berkov, F. Viladomat, C. Codina, J.A.S. Zuanazzi, and J. Bastida. 2011. Alkaloids from hippeastrum papilio. Molecules 16: 7097–7104.
Balkau, F., and M.L. Heffernen. 1973. NMR spectra of the carbolines. I. Spectral parameters. Australian Journal of Chemistry 26: 1501–1522.
Bandini, M., A. Eichholzer, M. Tragni, and A. Umani-Ronchi. 2008. Enantioselective phase-transfer-catalyzed intramolecular aza-michael reaction: Effective route to pyrazino-indole compounds. Angewandte Chemie 120: 3282–3285.
Bartus, R.T., R.R. Dean, B. Beer, and A.S. Lippa. 1982. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414.
Berkov, S., R. Reyes-Chilpa, C. Codina, F. Viladomat, and J. Bastida. 2007. Revised NMR data for incartine: An alkaloid from Galanthus elwesii. Molecules 12: 1430–1435.
Cowden, C.J., M.G. Banwell, and I.C. Ho. 1994. Synthesis of the putative structure of 5, 6-dihydrobicolorine. Journal of Natural Products 57: 1746–1750.
Eldeen, I.M., E.E. Elgorashi, and J. van Staden. 2005. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. Journal of Ethnopharmacology 102: 457–464.
Elgorashi, E.E., G.I. Stafford, and J. van Staden. 2004. Acetycholinesterase enzyme inhibitory effects of amaryllidaceae alkaloids. Planta Medica 70: 260–262.
Ellman, G.L., K.D. Courtney, V.J. Andres, and R.M. Feather-stone. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7: 88–95.
Evidente, A., I. Iasiello, and G. Randazzo. 1984. Hippamine, a minor alkaloid from Sternbergia lutea. Journal of Natural Products 47: 1061–1062.
Evidente, A., M. Rosaria Cicala, I. Giudicianni, G. Randazzo, and R. Riccio. 1983. 1H and 13c nmr analysis of lycorine and α-dihydrolycorine. Phytochemistry 22: 581–584.
He, Q., Y. Shen, M. Wang, M. Huang, R. Yang, S. Zhu, L. Wang, Y. Xu, and R. Wu. 2011. Natural variation in petal color in Lycoris longituba revealed by anthocyanin components. PLoS One 6: e22098.
Hiroko, S., H. Akihiro, and H. Tohru. 1993. The 1H- and 13C-nuclear magnetic resonance spectra of harman. Reinvestigation of the assignments by one- and two-dimensional methods. Chemical & Pharmaceutical Bulletin 41: 1169–1172.
Ieven, M., B.D.A. Vanden, F. Mertens, A. Vlietinck, and E. Lammens. 1979. Screening of higher plants for biological activities. I. Antimicrobial activity. Planta medica 36: 311–321.
Jin, A., X. Li, Y.Y. Zhu, H.Y. Yu, H.F. Pi, P. Zhang, and H.L. Ruan. 2014. Four new compounds from the bulbs of Lycoris aurea with neuroprotective effects against CoCl2 and H2O2-induced SH-SY5Y cell injuries. Archives of Pharmacal Research 37: 315–323.
Jin, J., and S.M. Weinreb. 1997. Application of a stereospecific intramolecular allenylsilane imino ene reaction to enantioselective total synthesis of the 5, 11-methanomorphanthridine class of amaryllidaceae alkaloids. Journal of the American Chemical Society 119: 5773–5784.
Jin, Z. 2005. Amaryllidaceae and sceletium alkaloids. Natural Product Reports 22: 111–126.
Jokhadze, M., L. Eristavi, J. Kutchukhidze, A. Chariot, L. Angenot, M. Tits, O. Jansen, and M. Frederich. 2007. In vitro cytotoxicity of some medicinal plants from Georgian Amaryllidaceae. Phytotherapy Research 21: 622–624.
Kihara, M., L. Xu, K. Konishi, K. Kida, Y. Nagao, S. Kobayashi, and T. Shingu. 1994. Isolation and structure elucidation of a novel alkaloid, incartion, a supposed biosynthetic intermediate, from flowers of Lycoris incarnata. Chemical & Pharmaceutical Bulletin 42: 289–292.
Kitagawa T., S. Uyeo, and N. Yokoyama. 1959. Stereochemistry of lycorenine, homolycorine, pluviine, and their hydrogenation products. Journal of the Chemical Society 3741–3751.
Kornienko, A., and A. Evidente. 2008. Chemistry, biology and medicinal potential of narciclasine and its congeners. Chemical Reviews 108: 1982–2014.
Li, L., J. Li, Y. Huang, Q. Wu, S. Deng, X. Su, R. Yang, J. Huang, Z. Chen, and S. Li. 2012. Lignans from the heartwood of Streblus asper and their inhibiting activities to Hepatitis B virus. Fitoterapia 83: 303–309.
Li, X., H.Y. Yu, Z.Y. Wang, H.F. Pi, P. Zhang, and H.L. Ruan. 2013. Neuroprotective compounds from the bulbs of Lycoris radiata. Fitoterapia 88: 82–90.
Li, Y., M. Zhao, and K.L. Parkin. 2011. β-Carboline derivatives and diphenols from soy sauce are in vitro quinone reductase (QR) inducers. Journal of Agricultural and Food Chemistry 59: 2332–2340.
Liang, Y.Q., X. Feng, and Z.X. Zhao. 2010. The alkaloids in bulbs of lycoris longituba. Natural Product Research and Development 22: 241–244.
Lopez, S., J. Bastida, F. Viladomat, and C. Codina. 2002. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and narcissus extracts. Life Sciences 71: 2521–2529.
Louw, C.A., T.J. Regnier, and L. Korsten. 2002. Medicinal bulbous plants of South Africa and their traditional relevance in the control of infectious diseases. Journal of Ethnopharmacology 82: 147–154.
Matusch, R., M. Kreh, M.U. Ller, and U. Bildung. 1994. Kristallstruktur und absolute Konfiguration von (-)-N-(Chloromethyl) galanthaminium-chlorid. Helvetica Chimica Acta 77: 1611–1615.
McNulty, J., J.J. Nair, C. Codina, J. Bastida, S. Pandey, J. Gerasimoff, and C. Griffin. 2007. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry 68: 1068–1074.
Pabuccuoglu V., P. Richomme, T. Gozler, B. Kivcak, A.J. Freyer, and M. Shamma. 1989. Four new crinine-type alkaloids from Sternbergia species. Journal of Natural Products 52: 785–791.
Pearson, W.H., and B.W. Lian. 1998. Application of the 2-azaallyl anion cyclo-addition method to an enantioselective total synthesis of (+)-coccinine. Angewandte Chemie 37: 1724–1726.
Perry, E.K. 1986. The cholinergic hypothesis—ten years on. British Medical Bulletin 42: 63–69.
Roberts, J.D., W.O. Crain Jr, and W.C. Wildman. 1971. Nuclear magnetic resonance spectroscopy. Carbon-13 spectra of nicotine, quinine, and some Amaryllidaceae alkaloids. Journal of the American Chemical Society 93: 990–994.
Sener, B., I. Orhan, and J. Satayavivad. 2003. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytotherapy Research 17: 1220–1223.
Senol, F.S., I. Orhan, G. Yilmaz, M. Cicek, and B. Sener. 2010. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food and Chemical Toxicology 48: 781–788.
Stanley, L.M., and J.F. Hartwig. 2009. Iridium-catalyzed regio- and enantioselective N-allylation of indoles. Angewandte Chemie 121: 7981–7984.
Suau, R., A.I. Gómez, and R. Rico. 1990. Ismine and related alkaloids from Lapiedra martinezii. 1712 29: 1710.
Treu, M., and U. Jordis. 2001. 4aS, 6R, 8aS)-4a, 5, 9, 10, 11, 12-Hexahydro-6H-benzofuro [3a, 3, 2-ef][2] benzazepine-3, 6-diol (Norsanguinine. Molecules 6: M274.
Tsuda, Y., T. Sano, J. Taga, K. Isobe, J. Toda, S. Takagi, M. Yamaki, M. Murata, H. Irie, and H. Tanaka. 1979. Total synthesis of the amaryllidaceae alkaloids, lycorine and zephyranthine. Journal of the Chemical Society, Perkin Transactions 1: 1358–1363.
Wang, L., Z. Yin, Y. Cai, X. Zhang, X. Yao, and W. Ye. 2010. Amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Biochemical Systematics and Ecology 38: 444–446.
Wu, W.M., Y.Y. Zhu, H.R. Li, H.Y. Yu, P. Zhang, H.F. Pi, and H.L. Ruan. 2014. Two new alkaloids from the bulbs of Lycoris sprengeri. Journal of Asian Natural Products Research 16: 192–199.
Zhao, Y., Y. Liang, Y. Chen, H. Sun, M. Wang, and X. Feng. 2011. Chemical constituents of bulbs of Lycoris longituba. Zhongyaocai 34: 1366–1368.
Acknowledgments
Financial supports from the Ministry of Science and Technology of the People’s Republic of China (International Cooperative Project, Grant No. 2010DFA32430) and Natural Science Foundation of China (No. 81072547, 31270394 and 30873361) are gratefully acknowledged.
Conflict of interest
All the authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, YY., Li, X., Yu, HY. et al. Alkaloids from the bulbs of Lycoris longituba and their neuroprotective and acetylcholinesterase inhibitory activities. Arch. Pharm. Res. 38, 604–613 (2015). https://doi.org/10.1007/s12272-014-0397-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0397-2