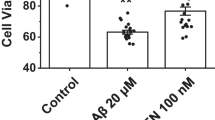
Abstract
Interaction of the Alzheimer’s Aβ peptides with the plasma membrane of cells in culture results in chronic increases in cytosolic [Ca2+]. Such increases can cause a variety of secondary effects leading to impaired cell growth or cell degeneration. In this investigation, we made a comprehensive study of the changes in cytosolic [Ca2+] in single PC12 cells and human neurons stressed by continuous exposure to a medium containing Aβ42 for several days. The differential timing and magnitude of the Aβ42-induced increase in [Ca2+] reveal subpopulations of cells with differential sensitivity to Aβ42. These results suggest that the effect produced by Aβ on the level of cytosolic [Ca2+] depends on the type of cell being monitored. Moreover, the results obtained of using potent inhibitors of Aβ cation channels such as Zn2+ and the small peptide NA7 add further proof to the suggestion that the long-term increases in cytosolic [Ca2+] in cells stressed by continuous exposure to Aβ is the result of Aβ ion channel activity.






Similar content being viewed by others
References
Abramov AY, Canevari L, Duchen MR (2003) Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci 23:5088–5095
Abramov AY, Canevari L, Duchen MR (2004) Calcium signals induced by amyloid β peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742:81–87
Aguayo LG, Parodi J, Sepúlveda FJ, Opazo C (2009) Pore-forming neurotoxin-like mechanism for Abeta oligomer-induced synaptic failure. In: Current Hypotheses and research Milestones in Alzheimer’s Disease (Perry G and Maccioni RB, eds) pp13-21. Springer
Arispe N (2004) Architecture of the Alzheimer’s AβP ion channel pore. J Membr Biol 197:33–48
Arispe N, Pollard HB, Rojas E (1993) Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [a beta P-(1–40)] in bilayer membranes. Proc Natl Acad Sci U S A 90:10573–10577
Arispe N, Pollard HB, Rojas E (1994a) β-amyloid Ca2+-channel hypothesis for neuronal death in Alzheimer disease. Mol Cell Biochem 140:119–125
Arispe N, Pollard HB, Rojas E (1994b) The ability of amyloid β-protein to form Ca2+ channels provides a mechanism for neuronal death in Alzheimer’s disease. Ann N Y Acad Sci 747:256–266
Arispe N, Pollard HB, Rojas E (1996) Zn2+ interaction with Alzheimer amyloid β protein calcium channels. Proc Natl Acad Sci U S A 93:1710–1715
Arispe N, Diaz J, Simakova O (2007) Aβ ion channels. Prospects for treating Alzheimer’s disease with Aβ channel blockers. Biochim Biophys Acta 1768:1952–1965
Arispe N, Diaz J, Durell SR, Shafrir Y, Guy HR (2010) Polyhistidine peptide inhibitor of the Aβ calcium channel potently blocks the Aβ-induced calcium response in cells. Theoretical modeling suggests a cooperative binding process. Biochemistry 49:7847–7853
Bathia R, Lin H, Lal R (2000) Fresh and globular amyloid β protein induces rapid cellular degeneration. A possible implication for calcium-uptake via AβP-channel. FASEB J 14:1233–1243
Bezprozvanny I (2009) Calcium signaling and neurodegenerative diseases. Trends Mol Med 15(3):89–100
Bezprozvanny IB (2010) Calcium signaling and neurodegeneration. Acta Nat 2(1):72–82
Bezprozvanny I, Mattson MP (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease.Trends in. Neurosciences 31(9):454–463
Bojarski L, Herms J, Kuznicki J (2008) Calcium dysregulation in Alzheimer’s disease. Neurochem Int 52:621–633
Chin JH, Tse FW, Harris K, Jhamandas JH (2006) Beta amyloid enhances intracellular calcium rises mediated by repeated activation of intracellular calcium stores and nicotinic receptors in acutely dissociated rat basal forebrain neurons. Brain Cell Biol 35(2–3):173–186
Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Membr Biol 280:17294–17300
Demuro A, Smith M, Parker I (2011) Single-channel Ca(2+) imaging implicates Aβ1-42 amyloid pores in Alzheimer’s disease pathology. J Cell Biol 195(3):515–24
Diaz JC, Linnhan J, Pollard HB, Arispe N (2006) Histidines 13 and 14 in the Aβ sequence are targets for inhibition of Alzheimer’s disease Aβ ion channel and cytotoxicity. Biol Res 39:447–460
Green KN, LaFerla FM (2008) Linking calcium to Abeta and Alzheimer’s disease. Neuron 59(2):190–194
Guo Q, Sebastian L, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP (1999) Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem 72(3):1019–1029
Johnson RD, Schauerte JA, Wisser KC, Gafni A, Steel DG (2011) Direct observation of single amyloid-β(1–40) oligomers on live cells: binding and growth at physiological concentrations. PLoS ONE 6(8):e23970. doi:10.1371/journal.pone.0023970
Kawahara M (2004) Disruption of calcium homeostasis in the pathogenesis of Alzheimer’s disease and other conformational diseases. Curr Alzheimer Res 1:87–95
Kawahara M, Kuroda Y (2000) Molecular mechanism of neurodegeneration induced by Alzheimer’s beta-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res Bull 53(4):389–397, Review
Kawahara M, Kuroda Y (2001) Intracellular calcium changes in neuronal cells induced by Alzheimer’s beta-amyloid protein are blocked by estradiol and cholesterol. Cell Mol Neurobiol 21(1):1–13
Kawahara M, Arispe N, Kuroda Y, Rojas E (1997) Alzheimer’s disease amyloid β-protein forms Zn2+-sensitive cation-selective channels across excited membrane patches from hypothalamic neurons. Biophys J 73:67–75
Kawahara M, Kuroda Y, Arispe N, Rojas E (2000) Alzheimer’s beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem 275(19):14077–14083
Kawahara M, Negishi-Kato M, Sadakane Y (2009) Calcium dyshomeostasis and neurotoxicity of Alzheimer’s beta-amyloid protein. Expert Rev Neurother 9(5):681–93
Khachaturian ZS (1987) Hypothesis on the regulation of cytosol calcium concentration and the aging brain, Neurobiol. Aging 8(1987):345–346
Khachaturian ZS (1994) Calcium hypothesis of Alzheimer’s disease and brain aging. Ann N Y Acad Sci 747:1–11
Korol TY, Korol SV, Kostyuk EP, Kostyuk PG (2008) Disruption of calcium homeostasis in Alzheimer’s disease. Neurophysiology 40(5/6):457–464
Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ (2008) Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59:214–225
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signaling in Alzheimer’s disease. Nat Rev Neurosci 3:862–872
Landfield PW (1987) ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol Aging 8:346–347
Lee G, Pollard HB, Arispe N (2002) Annexin 5 and apolipoprotein E2 protect against Alzheimer’s amyloid-β-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides 23(7):1249–1263
Lin H, Bhatia R, Lal R (2001) Amyloid protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB J 15:2433–2444
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430:631–639
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6:337–350
Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE (1992) beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci 12:376–389
Mattson MP, Tomaselli KJ, Rydel RE (1993) Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res 621(1):35–49
Negishi-Kato M, Kawahara M (2008) Neurosteroids block the increase in intracellular calcium level induced by Alzheimer’s β-amyloid protein in long-term cultured rat hippocampal neurons. Neuropsychiatr Dis Treat 4(1):209–18
Parodi J, Sepúlveda FJ, Roa J, Opazo C, Inestrosa NC, Aguayo LG (2010) Beta-amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J Biol Chem 285(4):2506–2514
Prangkio P, Yusko EC, Sept D, Yang J, Mayer M (2012) Multivariate analyses of amyloid-beta oligomer populations indicate a connection between pore formation and cytotoxicity. PLoS ONE 7(10):e47261. doi:10.1371/journal.pone.0047261
Ravin R, Blank PS, Steinkamp A, Rappaport SM, Ravin N et al (2012) Shear forces during blast, not abrupt changes in pressure alone, generate calcium activity in human brain cells. PLoS ONE 7(6):e39421. doi:10.1371/journal.pone.0039421
Schauerte JA, Wong PT, Wisser KC, Ding H, Steel DG, Gafni A (2010) Simultaneous single molecule fluorescence and conductivity studies reveal distinct classes of Aβ species on lipid bilayers. Biochemistry 49(14):3031–3039
Sciacca MF, Kotler SA, Brender JR, Chen J, Lee DK, Ramamoorthy A (2012) Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation. Biophys J 103:702–710
Sepulveda FJ, Parodi J, Peoples RW, Opazo C, Aguayo LG (2010) Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS ONE 5(7):e11820. doi:10.1371/journal.pone.0011820
Shirwany NA, Payette D, Xie J, Guo Q (2007) The amyloid beta ion channel hypothesis of Alzheimer’s disease. Neuropsychiatr Dis Treat 3(5):597–612
Simakova O, Arispe NJ (2006) Early and late cytotoxic effects of external application of the Alzheimer’s Abeta result from the initial formation and function of Abeta ion channels. Biochemistry 45:5907–5915
Simakova O, Arispe NJ (2007a) Alzheimer’s disease Aβ peptide binding to cell membranes is correlated to membrane surface phosphatidylserine levels. Biophys J 92(3):612a
Simakova O, Arispe NJ (2007b) The cell-selective neurotoxicity of the Alzheimer’s Aβ peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Aβ toxicity,”. J Neurosci 27(50):13719–13729
Simakova O, Arispe NJ. (2011) Fluorescent analysis of the cell-selective Alzheimer’s disease Aβ peptide surface membrane binding: influence of membrane components. Int J Alzheimers Dis. 2011;2011:917629. doi: 10.4061/2011/917629. Epub 2011 Jun 8
Small DH, Gasperini R, Vincent AJ, Hung AC, Foa L (2009) The role of Abeta-induced calcium dysregulation in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 16(2):225–33
Smith IF, Green KN, LaFerla FM (2005) Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium 38:427–437
Supnet C, Bezprozvanny I (2010) The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 47:183–189
Tofoleanu F, Buchete N-V (2012) Alzheimer Aβ peptide interactions with lipid membranes: fibrils, oligomers and polymorphic amyloid channels. Prion 6(4):339–45
Zhu YJ, Lin H, Lal R (2000) Fresh and nonfibrillar amyloid β protein(I-40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AβP-channel-mediated cellular toxicity. FASEB J 14:1244–1254
Acknowledgments
This work was supported by The Alzheimer’s Association of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, H., Arispe, N.J. Single-cell screening of cytosolic [Ca2+] reveals cell-selective action by the Alzheimer’s Aβ peptide ion channel. Cell Stress and Chaperones 20, 333–342 (2015). https://doi.org/10.1007/s12192-014-0551-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-014-0551-2