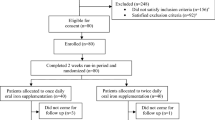
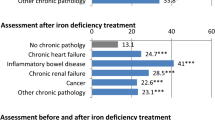
Abstract
It is difficult to predict the efficacy of deferasirox (DFX) as its pharmacokinetics varies among patients. The area under the curve (AUC) is reportedly useful for determining adequate DFX dosage; however, serum concentration measurements are often challenging. Effective DFX dosage is thus defined by assessing the efficacy of this agent in clinical practice. To analyze a predictive response marker to DFX therapy for use in adjusting the effective dosage during the early treatment phase, we retrospectively evaluated 39 DFX-treated patients. We defined response as a >40 % decrease in serum ferritin concentration from the pretreatment level. A maximum elevation of the total iron-binding capacity (TIBC) correlated with response in a multivariate analysis of iron metabolic markers (R 2 = 0.37, p < 0.001). A receiver operating characteristic curve analysis revealed that TIBC elevation had an AUC of 0.85 (p < 0.001) and the optimal cut-off value of TIBC elevation was 150 µg/dl. TIBC elevation of >150 µg/dl is a favorable predictor of effective ferritin reduction in DFX therapy (hazard ratio 29.6, 95 % confidence interval 4.8–183.6; p < 0.001). DFX therapy with TIBC monitoring may enable the determination of the minimum effective DFX dosage.



Similar content being viewed by others
References
Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematol Am Soc Hematol Educ Program. 2001;1:47–61.
Kikuchi S, Kobune M, Iyama S, Sato T, Murase K, Kawano Y, et al. Prognostic significance of serum ferritin level at diagnosis in myelodysplastic syndrome. Int J Hematol. 2012;95:527–34.
Cohen AR. New advances in iron chelation therapy. Hematol Am Soc Hematol Educ Program. 2006;1:42–7.
Cappellini MD, Porter J, El-Beshlawy A, Li CK, Seymour JF, Elalfy M, et al. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010;95:557–66.
Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with B-thalassemia. Blood. 2006;107:3455–62.
Vichinsky E, Onyekwere O, Porter J, Swerdlow P, Eckman J, Lane P, et al. A randomized comparison of deferasirox versus deferoxamine for the therapy of transfusional iron overload in sickle cell disease. Br J Haematol. 2006;136:501–8.
Gattermann N, Finelli C, Porta MD, Fenaux P, Ganser A, Guerci-Bresler A, et al. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: Results from the large 1-year EPIC study. Leuk Res. 2010;34:1143–50.
Taher A, Cappellini MD, Vinchinsky E, Galanello R, Piga A, Lawniczek T, et al. Efficacy and safety of deferasirox dosage of >30 mg/kg per d in patients with transfusion-dependent anemia and iron overload. Br J Haematol. 2009;147:752–9.
Lee JW, Yoon SS, Shen ZX, Ganser A, Hsu HC, Habr D, et al. Iron chelating therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448–54.
Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, et al. Relative response of patients with myelodysplastic syndromes and other transfusion dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2007;80:168–76.
Porter JB, Elalfy MS, Taher AT, Aydinok Y, Chan LL, Lee SH, et al. Efficacy and safety of deferasirox at low and high iron burdens: results from the EPIC magnetic resonance imaging substudy. Ann Hematol. 2013;92:211–9.
Metzgeroth G, Dinter D, Schultheis B, Dorn-Beineke A, Lutz K, Leismann O, et al. Deferasirox in MDS patients with transfusion-caused iron overload-a phase-2 study. Ann Hematol. 2009;88:301–10.
Chirnomas D, Smith AL, Braunstein J, Finkelstein Y, Pereira L, Bergmann AK, et al. Deferasirox pharmacokinetics in patients with adequate versus inadequate response. Blood. 2009;114:4009–13.
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology pf iron overload. Int J Hematol. 2008;88:7–15.
Crichton RR, Charloteauc-Wauters M. Iron transport and storage. Eur J Biochem. 1987;164:485–506.
Ikuta K, Ito S, Tanaka H, Sasaki K, Torimoto Y, Kohgo Y. Interference of deferasirox with assays for serum iron and serum unsaturated iron binding capacity during iron chelating therapy. Clin Chim Acta. 2011;412:2261–6.
Exjade. Highlight of prescribing information. https://www.pharma.us.novartis.com/product/pi/pdf/exjade.pdf
Miyazawa K, Ohyashiki K, Urabe A, Hata T, Nakao S, Ozawa K, et al. A safety, pharmacokinetic and pharmacodynamics investigation of deferasirox (Exjade, ICL670) in patients with transfusion-dependent anemias and iron overload: a phase I study in Japan. Int J Hematol. 2008;88:73–81.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Watanabe, J., Sato, K., Horiuchi, T. et al. Elevated total iron-binding capacity as a predictor of response to deferasirox therapy in the setting of chronic iron overload. Int J Hematol 100, 254–259 (2014). https://doi.org/10.1007/s12185-014-1624-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1624-9