Abstract
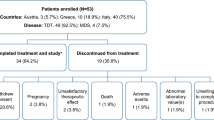
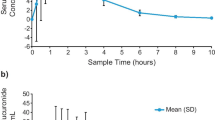
The pharmacokinetics (PK) and pharmacodynamics (PD) of the once-daily, oral ironchelating agent, deferasirox (Exjade®, ICL670), have been evaluated further in a Phase I, openlabel, multicenter, dose-escalation study in Japanese patients with myelodysplastic syndromes, aplastic anemia, and other anemias. Deferasirox was initially administered as a single dose of 5 (n = 6), 10 (n = 7), 20 (n = 6) or 30 (n = 7) mg/(kg day) and then after 7 days seven daily doses were administered. Linear PK (C max and AUC) were observed at all doses after a single dose and at steady state, and dose-dependent iron excretion was observed. Pharmacokinetic/pharmacodynamic parameters were similar to those reported in a Caucasian β-thalassemia cohort. Following the single- and multiple-dose phases, 21 of 26 patients progressed to a 3-year extension phase of the study, where dose reductions and increases [5–30 mg/(kg day)] were allowed following safety and efficacy assessments. In the interim, 1-year data show that deferasirox was well tolerated, with generally infrequent and mild adverse events. Reductions in serum ferritin levels were observed and a negative iron balance achieved at doses of 20–30 mg/(kg day). These data suggest that deferasirox has a stable and predictable PK/PD profile, irrespective of underlying disease or race, and a predictable and manageable safety profile suitable for chronic administration.






Similar content being viewed by others
References
Olivieri N. Thalassaemia: clinical management. Baillieres Clin Haematol. 1998;11:147–62.
Jansen AJ, Essink-Bot ML, Beckers EA, Hop WC, Schipperus MR, Van Rhenen DJ. Quality of life measurement in patients with transfusion-dependent myelodysplastic syndromes. Br J Haematol. 2003;121:270–4.
Lee MT, Piomelli S, Granger S, et al. Stroke prevention trial in sickle cell anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–52.
McLaren GD, Muir WA, Kellermeyer RW. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci. 1983;19:205–66.
Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology Am Soc Hematol Educ Program. 2001;47–61.
Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95:26–36.
Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous β-thalassemia. N Engl J Med. 1994;331:574–8.
Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–61.
Cortelezzi A, Cattaneo C, Cristiani S, et al. Non-transferrin-bound iron in myelodysplastic syndromes: a marker of ineffective erythropoiesis? Hematol J. 2000;1:153–8.
Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–13.
Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–87.
Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78:487–94.
Gattermann N, Porter JB, Lopes LF, Seymour J. Iron overload in myelodysplastic syndromes. Hematol Oncol Clin North Am. 2005;19 Suppl 1:18–25.
Piga A, Galanello R, Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–80.
Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80:168–76.
Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood. 2006;107:3455–62.
Vichinsky E, Onyekwere O, Porter J, et al. A randomized comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136:501–8.
Galanello R, Piga A, Forni GL, et al. Phase II clinical evaluation of deferasirox, a once-daily oral chelating agent, in paediatric patients with β-thalassaemia major. Haematologica. 2006;91:1343–51.
Nisbet-Brown E, Olivieri NF, Giardina PJ, et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361:1597–602.
Piga A, Galanello R, Cappellini MD, et al. Phase II study of ICL670, an oral chelator, in adult thalassaemia patients with transfusional iron overload: efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) after 18 months of therapy. Blood. 2003;102: abstr 412.
Vichinsky E. Clinical application of deferasirox: practical patient management. Am J Hematol. 2008;83:398–402.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Miyazawa, K., Ohyashiki, K., Urabe, A. et al. A safety, pharmacokinetic and pharmacodynamic investigation of deferasirox (Exjade®, ICL670) in patients with transfusion-dependent anemias and iron-overload: a Phase I study in Japan. Int J Hematol 88, 73–81 (2008). https://doi.org/10.1007/s12185-008-0115-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-008-0115-2