Abstract
Seven types of activated carbon were used to investigate the effect of their structure on separation of CO2 from (H2 + CO2) gas mixture by the adsorption method at ambient temperature and higher pressures. The results showed that the limiting factors for separation of CO2 from 53.6 mol% H2 + 46.4 mol% CO2 mixture and from 85.1 mol% H2 + 14.9 mol% CO2 mixture were different at 20 °C and about 2 MPa. The best separation result could be achieved when the pore diameter of the activated carbon ranged from 0.77 to 1.20 nm, and the median particle size was about 2.07 µm for 53.6 mol% H2 + 46.4 mol% CO2 mixture and 1.41 µm for 85.1 mol% H2 + 14.9 mol% CO2 mixture. The effect of specific area and pore diameter of activated carbon on separation CO2 from 53.6 mol% H2 + 46.4 mol% CO2 mixture was more significant than that from 85.1 mol% H2 + 14.9 mol% CO2 mixture. CO2 in the gas phase can be decreased from 46.4 mol% to 2.3 mol%–4.3 mol% with a two-stage separation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen is becoming an important energy carrier to meet the needs of both the chemical industry and electrical generation (Momirlan and Veziroglu 2005; Yang et al. 2006). Hydrogen has two key advantages: low emissions and high calorific value. On the other hand, the synthetic gas originating from the steam reforming of natural gas followed by the water gas shift reaction is composed of (CO2 and H2) and a large amount of hydrogen can be obtained by purification of the synthetic gas (Hufton et al. 1999; Lee et al. 2007). Thus far, due to its merits of low cost, low-energy requirement, low pressure, and modest temperature, the method of adsorption separation has recently been received much interest. The critical factor for the method of adsorption separation is to find a suitable adsorbent with high selectivity for CO2 and high CO2 adsorption capacity.
There are several studies demonstrating that to maximise CO2 uptake carbon materials need to have suitable structural features. Wickramaratne and Jaroniec (2013) synthesized phenolic resin-based carbon spheres obtained by a slightly modified Stober method, and found that it showed microporous carbon with fine pores (< 1 nm) and high specific surface area (2400 m2/g) has high CO2 capture at 0 °C and ambient pressures. The results of Yin et al. (2013) indicated that the surface properties of activated carbon had a little effect on CO2 adsorption, while the volume of ultra-micropore (< 0.7 nm) had a significant effect on excess CO2 adsorption capacity at pressure swing adsorption (PSA) conditions (0–20 °C, 0.01–0.10 MPa). Liu et al. (2017) synthesized nitrogen-enriched porous carbon spheres through a one-pot carbonization process and they proved that the carbon spheres which had a large amount of small micropores (< 1.0 nm) showed a good capacity to store CO2 at 0.1 MPa and 25 °C. Adeniran et al. (2014) synthesized microporous carbons by heating a high carbon-containing metal salt under nitrogen, and their experimental results showed that 0.6–0.7 nm was advantageous for CO2 uptake in a CO2/N2 system at 0.1 MPa and 25 °C. Kwac et al. (2016) emphasized the importance of graphitic pore size from 0.8 to 1.0 nm in CO2 capture and selectivity against N2. Coromina et al. (2015) found carbon with small micropores could satisfy the requirements of CO2 uptake at low pressure and carbon with high surface area could satisfy the requirements for high pressure. In addition, there is some work (Cox and Mokaya 2017; Cao and Wu 2005) about the limiting factor for separating CO2 from H2 using carbon materials. Cox and Mokaya (2017) demonstrated that the porosity of the carbons, i.e., mesopores of size 2.5–5.0 nm and a few micropores, was favorable for CO2 uptake at 25 °C and a pressure of 2–5 MPa, and their finding suggested that the presence of microporosity was a limiting factor in the CO2 uptake capacity especially at high pressure (3–5 MPa). Cao and Wu (2005) simulated the separation of H2/CO2 via adsorption in activated carbon using grand canonical Monte Carlo simulations at 25 °C and 1–8 MPa and identified that the optimum pore sizes for the bulk mole fraction ratio of \(x_{{{\text{CO}}_{2} }} /x_{{{\text{H}}_{2} }}\) = 1:2 and \(x_{{{\text{CO}}_{2} }} /x_{{{\text{H}}_{2} }}\) = 1:8 were 1.48 nm and 1.18 nm, respectively.
In conclusion, a lot of work has been done on the limiting factors of CO2 capture and storage with carbon materials, and a little experimental work has been done for the separation of (H2 + CO2) mixtures with activated carbon under high pressures. Considering the significance of structural features of the adsorbent for the selectivity of separating (H2 + CO2) mixtures with the adsorption method, it is necessary to study the effect of the structural features on separation performance of carbon materials at higher pressure. In this work, we first compared the selectivity for a (H2 + CO2) mixture using activated carbon KC-88 with those using metal–organic frameworks (MOFs) or membranes. Then, the effects of the specific surface area, micropore volume, pore diameter, and median particle size of the activated carbons were investigated for separating gas mixtures of (53.6 mol% H2 + 46.4 mol% CO2) and (85.1 mol% H2 + 14.9 mol% CO2) at about 2 MPa. The reusability of the activated carbons was also examined. These experimental data are useful for future applications of the separation of H2/CO2 gas mixtures with activated carbon or other porous materials at higher pressures.
2 Experimental section
2.1 Apparatus and material
The experimental apparatus used in this work has been described in detail in our previous papers (Liang et al. 2005; Zhang et al. 2014), and the schematic diagram is shown in Fig. 1.
Hydrogen (99.99%) and carbon dioxide (99.99%) were obtained from Beijing AP Beifen Gases Industry Company Limited. The typical synthesis gas from an integrated gasification combined cycle (IGCC) power station consists of approximately 60 mol% H2 and 40 mol% CO2 (Klara and Srivastava 2002; Jebraeel et al. 2011); a gas mixture of (53.6 mol% H2 + 46.4 mol% CO2) was prepared in our laboratory as the first component of feed gas. To observe the difference of separation performance in systems with different mole fraction ratios of CO2 and the separation performance of activated carbon through a two-stage separation process, a gas mixture of (85.1 mol% H2 + 14.9 mol% CO2) was prepared as the second example of feed gas. The compositions of the feed gas and equilibrium gas were analyzed by a Hewlett-Packard 7890 gas chromatograph. The distilled water used has a conductivity of less than 10−4 S m−1. Activated carbons were purchased from Hainuo Carbon Industry Co., Ltd., Shanghai (China), and Kecheng Guanghua New Technology Co., Ltd., Beijing (China). Before the experiment, the activated carbon samples (HN-1, HN-2, HN-3, HN-4, KC-92, KC-88, and Sigma) were dried to constant mass in a vacuum drying oven at 100 °C. The scanning electron microscope images of HN-1, HN-2, HN-3, HN-4, KC-92, KC-88, and Sigma were obtained from an FEI Quanta 200F SEM. The corresponding properties such as the specific surface area, micropore volume, and pore diameter, for these seven types of activated carbon were measured on a Micrometrics ASAP 2020 HD88 accelerated surface area and porosimetry system, and their median size and skeletal density were measured with a HORIBA LA-950 laser particle size analyzer and a Quantachrome Ultrapycnometer 1000, respectively.
2.2 Procedures and data processing
The separation performance of activated carbons was evaluated according to the following procedure. At first, the sapphire cell was dismounted from the apparatus, washed with distilled water, dried, and loaded with a certain mass of activated carbon. Subsequently, the cell was installed into the apparatus again and connected to the blind cell, and the whole system was evacuated. Afterward, the top valve of the sapphire cell was closed and the blind cell was charged with the feed gas until the desired pressure was achieved. The air-bath temperature was then regulated to the desired value. When the temperature and the pressure of the gas mixture in the blind cell were stable at the specified value for a period of time, the stable pressure of the blind cell was recorded as P1. Thereafter, the top valve of the sapphire cell was opened and the desired amount of feed gas originating from the blind cell was injected into the sapphire cell. When the pressure of the sapphire cell reached the desired value (recorded as P0), the top valve was closed. Then, the adsorption separation experiment took place in the sapphire cell. After the pressure of the sapphire cell remained constant for a period of time, the separation process was deemed to have ended. The pressure of the residual gas in the blind cell and in the sapphire cell was recorded as P2 and PE, respectively. The equilibrium gas mixture in the sapphire cell was sampled under constant pressure by pushing the piston using a hand pump, and the corresponding composition was analyzed by HP 7890 gas chromatography.
Using the above procedures, the separation performance of activated carbons with different structures and the repeatability of activated carbons were investigated. The obtained data were processed as follows.
The total number of moles of the gas mixture (nt) injected into the sapphire cell was calculated by the following formula:
where P1 was the initial pressure of the blind cell and P2 was the residual pressure of the blind cell after injecting a certain amount of gas into the sapphire cell; Vt was the total volume of the blind cell plus the tubes connected to two cells; T was the system temperature; R was the universal gas constant; and the compressibility factors Z1, Z2 were calculated using the Benedict–Webb–Rubin–Starling equation of state.
The total number of moles of the equilibrium gas phase (nE) in the sapphire cell was calculated by the following formula:
where PE was the equilibrium pressure of the sapphire cell; Vg was the volume of the equilibrium gas in the sapphire cell; ZE was the compressibility factor corresponding to T, PE, and the gas phase composition in the sapphire cell. The volume of the equilibrium gas in the sapphire cell was calculated by the following:
where VS was the effective volume of the sapphire cell and VC was the skeleton volume of the activated carbon, which was further defined as follows:
where mc was the mass of the activated carbon for the separation experiments and \(\rho_{\text{s}}\) was the skeletal density of the activated carbon.
The total adsorbed number of moles of H2 (n1) and CO2 (n2) in the fixed bed of the activated carbon were calculated by the following::
where zi and yi were the molar fraction of H2 and CO2 in the feed gas and in the equilibrium gas phase, respectively. In addition, the corresponding molar fractions of H2 and CO2 in the pores of the activated carbon were defined as follows:
The initial gas–solid ratio was calculated by the following:
The efficiency of separation was represented by selectivity, β, defined by the following (Cao and Wu 2005; Yang et al. 2009):
The recovery of CO2 in the adsorbed phase, R2, was calculated by the following:
The adsorption capacity for CO2, M2, was calculated by the following:
3 Results and discussion
3.1 Comparison of the separation effect of different materials
The selectivities of H2/CO2 separation with different materials were first compared. The separation experiment with activated carbon KC-88 at 25 °C (named as run 1) was performed in this work with the feed gas composition of (53.6 mol% H2 + 46.4 mol% CO2), and the experimental results are tabulated in Table 1. For comparison, the simulated or experimental results with MOFs (Yang et al. 2009; Keskin and Sholl 2009), activated carbon (Cao and Wu 2005), or membranes (Richard et al. 2001; Guo et al. 2009) in the literature are also listed in Table 1, while the composition of the feed gas used in the literature is (50 mol% H2 + 50 mol% CO2). It can be seen that the selectivity, β, for MOF-5 (Keskin and Sholl 2009) and isoreticular metal–organic frameworks (IRMOFs) (Yang et al. 2009) (IRMOF-9, IRMOF-10, IRMOF-12, IRMOF-13, and IRMOF-14) are less than 100, while the selectivity, β, for membranes (microporous silica membrane and HKUST-1 membrane) (Richard et al. 2001; Guo et al. 2009) are less than 10 when the equilibrium pressure is approximately 1 MPa at 25 °C. The selectivity, β, in activated carbon (Cao and Wu 2005) is 45 at 25 °C and 2 MPa. However, for the activated carbon KC-88 used in this work, the selectivity, β, reaches 211 when the equilibrium pressure is 0.90 MPa at 25 °C. It is known that the price of MOFs and IRMOFs for use in separation experiments is very high and these materials are scarce. In comparison, the cost of activated carbon is very low and the production technology of activated carbon is so mature that it can be bought easily. However, there exist different structural features for different activated carbons. Suitable activated carbon that has a higher separation factor value must be chosen.
3.2 Effect of the structure of activated carbon
To examine the effect of the structure of activated carbon on the separation performance for (H2 + CO2) gas mixtures, seven types of activated carbon, namely HN-1, HN-2, HN-3, HN-4, KC-92, KC-88, and Sigma, were evaluated. The SEM images and their particle distribution are shown in Figs. 2, 3, and 4. The corresponding properties such as the specific surface area, micropore volume, pore diameter, median particle size, and skeletal density for the activated carbons are shown in Table 2. Among these activated carbons, HN-1, HN-2, HN-3, and HN-4 are derived from the same type of activated carbon, and have the same specific surface area, micropore volume, pore diameter, and skeletal density but with different particle sizes. The median particle sizes are 1.14, 1.41, 2.07, and 6.18 µm for HN-1, HN-2, HN-3, and HN-4, respectively. It is known that, in activated carbon, the particle size decides the utilization rate of the micropores, the pore diameter controls the probability of the gas molecules entering the pores of activated carbon, and the specific surface area and the pore volume decide the storage capacity for gas (Ozdemir and Schroeder 2009). That is, an optimum value exists for the pore diameter and the particle size to separate (H2 + CO2) gas mixtures, but, for the pore volume or specific surface area, the larger the better CO2 uptake. In this work, two gas mixtures were chosen to evaluate the effect of activated carbon’s structure on the separation performance, which was (53.6 mol% H2 + 46.4 mol% CO2) and (85.1 mol% H2 + 14.9 mol% CO2). The separation results of seven types of activated carbon with these two feed gas mixtures (runs 2–15) are tabulated in Table 3. For this series of experiments, the temperature and initial pressure were 10 °C and approximately 2.0 MPa, respectively.
The separation performance for the (53.6 mol% H2 + 46.4 mol% CO2) feed gas mixture is first discussed. For HN-1, HN-2, HN-3, or HN-4 in which only the median particle size is different (runs 2–5), with the increasing particle size from 1.14 to 6.18 µm, the molar fraction of CO2 in the equilibrium gas mixture, y2, reached the minimum value (15.3%) and the adsorption capacity, M2, for CO2 reached maximum value (3.3 mmol/g) when the median particle size was 1.41 µm (HN-2); the selectivity, β, reached the maximum value (161) when the median particle size was 2.07 µm (HN-3). The experimental results showed that the median particle size controlled the CO2 capture and selectivity for (53.6 mol% H2 + 46.4 mol% CO2) mixture when other structural features were constant, and the median particle size for optimal CO2 capture and for optimal selectivity were different. Comparing the separation results of HN-1, HN-2, HN-3, and HN-4 for the (85.1 mol% H2 + 14.9 mol% CO2) (runs 9–15), y2 reached the minimum value (2.9%) and the selectivity, β, reached the maximum value (181) when the median particle size was 1.41 µm; with the increasing of the particle size, M2 changed slightly. Comparing the separation performance of HN-1, HN-2, HN-3, and HN-4 for (53.6 mol% H2 + 46.4 mol% CO2) and (85.1 mol% H2 + 14.9 mol% CO2), the effect of the particle size was more important for selectivity than CO2 capture when the concentration of CO2 was low.
Comparing the separation performance of KC-92, KC-88, and Sigma for (53.6 mol% H2 + 46.4 mol% CO2), y2 reached a minimum value (12.2%), the selectivity β, and M2 reached a maximum value (β = 149, M2 = 3.5 mmol/g) for KC-88. Through comparing the structural information for KC-92, KC-88, and Sigma in Table 2, it could be probably confirmed that the specific surface area of KC-88 (1716 m2/g) prompted y2 to reach the minimum value (12.2%) and prompted M2 to reach the maximum value (3.5 mmol/g), and the pore diameter (1.20 nm) or median particle size (6.02 µm) for KC-88 made selectivity reach the maximum value (β = 149). Comparing the structural features of HN-4 and KC-88, the median particle size of HN-4 (6.18 µm) and KC-88 (6.02 µm) were almost the same; the specific surface area and the pore diameter for HN-4 (425 m2/g, 0.77 nm) and KC-88 (1716 m2/g, 1.20 nm) differed greatly. Combining the difference of structural features for HN-4 and KC-88, the separation performance of HN-4 and KC-88 showed that the specific surface area decided the adsorption capacity for CO2 and the pore diameter limited the selectivity for (53.6 mol% H2 + 46.4 mol% CO2), which was identical with the works of Cox and Mokaya (2017) about the presence of microporosity limiting the separation performance at higher pressure. Combining difference of the selectivity for HN-1, HN-2, HN-3, and HN-4 with increasing median particle size, the median particle size limited the selectivity of activated carbon for (53.6 mol% H2 + 46.4 mol% CO2). In conclusion, for (53.6 mol% H2 + 46.4 mol% CO2), the specific surface area limited the CO2 capture, and the median particle size and the pore diameter limited the selectivity. The best separation performance is attained when the pore diameter is in the range of 0.77–1.20 nm and the median particle size is about 2.07 µm.
Table 3 also lists the separation performance of the activated carbons with the feed gas mixture of (85.1 mol% H2 + 14.9 mol% CO2) (runs 9–15). Comparing the separating performance of HN-4 and KC-88, the specific surface area and the pore diameter limited y2 and selectivity β when median particle size was almost same. Comparing the separation performance of HN-3 and KC-92, y2 and selectivity β were almost the same (HN-3: 3.0%, 82; KC-92: 3.1%, 81) when the specific surface area differed greatly (HN-3: 425 m2/g and KC-92: 1442 m2/g). Combining the variation of β, the median particle size limits the selectivity of activated carbon for (85.1 mol% H2 + 14.9 mol% CO2), and the best performance is reached when the median particle size is about 1.41 µm. Comparing the variation of y2 and M2 for seven activated carbon, the effect of specific area and pore diameter for (85.1 mol% H2 + 14.9 mol% CO2) are weaker than (53.6 mol% H2 + 46.4 mol% CO2), and better CO2 capture can be reached when the pore diameter is in the range of 0.77–1.20 nm.
In conclusion, the best performance of (53.6 mol% H2 + 46.4 mol% CO2) and (85.1 mol% H2 + 14.9 mol% CO2) can be reached when the pore diameter is in the range of 0.77–1.20 nm and the median size is about 2.07 µm and 1.41 µm, respectively. Comparing the simulation results of Cao and Wu (2005) who have confirmed that the optimized pore sizes for the bulk mole fraction ratio of \(x_{{{\text{CO}}_{2} }} /x_{{{\text{H}}_{2} }}\) = 1:2 and \(x_{{{\text{CO}}_{2} }} /x_{{{\text{H}}_{2} }}\) = 1:8 at 25 °C and 1–8 MPa are 1.48 nm and 1.18 nm, respectively, the experimental results in this work at 10 °C and about 2 MPa are reliable.
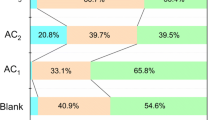
In addition, the feed gas composition of (85.1 mol% H2 + 14.9 mol% CO2) in runs 9–15 can be regarded as the composition of an equilibrium gas phase for feed gas (53.6 mol% H2 + 46.4 mol% CO2) in runs 2–8 after one stage of adsorption process. Therefore, the percent of CO2 in the gas mixture of (53.6 mol% H2 + 46.4 mol% CO2) can be reduced to less than 4.3% after two stages of the adsorption process using activated carbon.
3.3 Regeneration of the activated carbon
To investigate the reuse of activated carbon for adsorption separation, separation experiments with KC-88 which was regenerated at different conditions with feed gas mixture of (53.6 mol% H2 + 46.4 mol% CO2) were performed at 10 °C and an initial pressure of 2.1 MPa. For run 7, fresh KC-88 was used; for run 16, KC-88 was regenerated by evacuating at 20 °C for 10 h; for runs 17–21, KC-88 was regenerated at different temperatures (20, 40, 60, 80, and 100 °C, respectively). The separation results for these experiments with the regenerated KC-88 are tabulated in Table 4. As observed from Table 4, for run 16, the molar fraction of CO2 in the equilibrium gas y2 reached 15.0% and the selectivity β reached 120, which was close to those with the fresh activated carbon system (run 7). For runs 17–21 with the regeneration temperature increasing from 20 to 100 °C, y2 decreased from 23.89% to 12.3%, β increased from 21 to 138, x2 increased from 86.9% to 95.0%, R2 increased from 62.2% to 82.6%, and M2 increased from 2.38 to 3.24 mmol/g. The separation performance of run 21 was similar to that of run 7. That is, the higher regeneration temperature is good for regenerating the activated carbon to be reused in subsequent separation experiments. Comparing the separation results of runs 16 and 20, y2 in run 16 (15.0%) was almost the same as that in run 20 (14.5%), but x2 and β in run 16 (x2 = 95.5%, β = 120) were better than those in run 20 (x2 = 92.6%, β = 74). Considering the separation results and the cost to regenerate the activated carbons with two different methods, it can be concluded that the used activated carbon is better regenerated by evacuating rather than by heating.
4 Conclusion
Seven types of activated carbon were examined to determine the limiting factors of the structure of activated carbons for separating gas mixtures (53.6 mol% H2 + 46.4 mol% CO2) and (85.1 mol% H2 + 14.9 mol% CO2) at 10 °C and about 2 MPa. The experimental data show that: for 53.6 mol% H2 + 46.4 mol% CO2, the pore diameter and median particle size limit the selectivity of activated carbons; for 85.1 mol% H2 + 14.9 mol% CO2, the median particle size limits the selectivity of activated carbons; the best separation performance can be attained for the two kinds of gas mixture when the pore diameter of the activated carbon ranges from 0.77 to 1.20 nm, and the median particle size is about 2.07 and 1.41 µm, respectively; the effect of specific area and pore diameter for latter mixture are weaker than the former mixture.
In addition, the molar fraction of CO2 in the equilibrium gas mixture can be reduced from 46.4% to less than 4.3% in two stages using activated carbon. Over all, our findings are beneficial for separating industrial gas mixtures containing CO2 using activated carbons under ambient temperature and higher pressures.
References
Adeniran B, Masika E, Mokaya R. A family of microporous carbons prepared via a simple metal salt carbonization route with high selectivity for exceptional gravimetric and volumetric post-combustion CO2 capture. J Mater Chem A. 2014;35:14696–710. https://doi.org/10.1039/C4TA03565H.
Cao DP, Wu JZ. Modeling the selectivity of activated carbons for efficient separation of hydrogen and carbon dioxide. Carbon. 2005;43:1364–70. https://doi.org/10.1016/j.carbon.2005.01.004.
Coromina HM, Walsh DA, Mokaya R. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications. J Mater Chem A. 2015;4:280–9. https://doi.org/10.1039/c5ta09202g.
Cox M, Mokaya R. Ultra-high surface area mesoporous carbons for colossal pre-combustion CO2 capture and storage as materials for hydrogen purification. Sustain Energy Fuels. 2017;1:1414–24. https://doi.org/10.1039/c7se00300e.
Guo H, Zhu G, Hewitt IJ, et al. “Twin Copper Source” growth of metal organic framework membrane: Cu3(BTC)2 with high permeability and selectivity for recycling H2. J Am Chem Soc. 2009;131:1646–7. https://doi.org/10.1021/ja8074874.
Hufton JR, Mayorga S, Sircar S. Sorption-enhanced reaction process for hydrogen production. AIChE J. 1999;45:248–56. https://doi.org/10.1002/aic.690450205.
Jebraeel G, Antonin C, Bahman T. Separation and capture of carbon dioxide from CO2/H2 syngas mixture using semi-clathrate hydrates. Chem Eng Res Des. 2011;89:1747–51. https://doi.org/10.1016/j.cherd.2011.03.008.
Keskin S, Sholl DS. Assessment of a metal organic framework membrane for gas separations using atomically detailed calculations: CO2, CH4, N2, H2 mixtures in MOF-5. Ind Eng Chem Res. 2009;48:914–22. https://doi.org/10.1021/ie8010885.
Klara SM, Srivastava RD. US DOE integrated collaborative technology development program for CO2 separation and capture. Environ Prog Sustain Energy. 2002;21:247–53. https://doi.org/10.1002/ep.670210414.
Kwac K, Lee JH, Choi JW, et al. Computational analysis of pressure-dependent optimal pore size for CO2 capture with graphitic surfaces. J Phys Chem C. 2016;120:3978–85. https://doi.org/10.1021/acs.jpcc.5b12404.
Lee KB, Beaver MG, Caram HS, et al. Novel thermal-swing sorption-enhanced reaction process concept for hydrogen production by low-temperature steam-methane reformation. Ind Eng Chem Res. 2007;46:5003–14. https://doi.org/10.1021/ie0701064.
Liang MY, Chen GJ, Sun CY, et al. Experimental and modeling study on decomposition kinetics of methane hydrates in different media. J Phys Chem B. 2005;109:19034–41. https://doi.org/10.1021/jp0526851.
Liu L, Xie ZH, Deng QF, et al. One-pot carbonization enrichment of nitrogen in microporous carbon spheres for efficient CO2 capture. J Mater Chem A. 2017;5:418–25. https://doi.org/10.1039/C6TA0978K.
Momirlan M, Veziroglu TN. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int J Hydrog Energy. 2005;30:795–802. https://doi.org/10.1016/j.ijhydene.2004.10.011.
Ozdemir E, Schroeder K. Effect of moisture on adsorption isotherms and adsorption capacities of CO2 on coals. Energy Fuels. 2009;23:2821–31. https://doi.org/10.1021/ef801126a.
Richard V, Favre E, Tondur D, et al. Experimental study of hydrogen, carbon dioxide and nitrogen permeation through a microporous silica membrane. Chem Eng J. 2001;84:593–8. https://doi.org/10.1016/S1385-8947(01)00173-5.
Wickramaratne NP, Jaroniec M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J Mater Chem A. 2013;1:112–6. https://doi.org/10.1039/C2TA00388K.
Yang QY, Xu Q, Liu B, et al. Molecular simulation of CO2/H2 mixture separation in metal-organic frameworks: effect of catenation and electrostatic interactions. Chin J Chem Eng. 2009;17:781–90. https://doi.org/10.1016/S1004-9541(08)60277-3.
Yang YZ, Chang CH, Idriss H. Photo-catalytic production of hydrogen from ethanol over M/TiO2 catalysts (M = Pd, Pt or Rh). Appl Catal B. 2006;67:217–22. https://doi.org/10.1016/j.apcatb.2006.05.007.
Yin GJ, Liu ZY, Liu QY, et al. The role of different properties of activated carbon in CO2 adsorption. Chem Eng J. 2013;230:133–40. https://doi.org/10.1016/j.dej.2013.06.085.
Zhang XX, Liu H, Sun CY, et al. Effect of water content on separation of CO2/CH4 with active carbon by adsorption-hydration hybrid method. Sep Purif Technol. 2014;130:132–40. https://doi.org/10.1016/j.seppur.2014.04.028.
Acknowledgements
Financial support was received from the Talent Scientific Research Fund of LSHU (No. 2016XJJ-015), the fund of the Liaoning Provincial Department of Education (No. L2017LQN005), and the National Natural Science Foundation of China (No. 21606120).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Xiu-Qin Zhu
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, XX., Xiao, P., Sun, CY. et al. Optimal activated carbon for separation of CO2 from (H2 + CO2) gas mixture. Pet. Sci. 15, 625–633 (2018). https://doi.org/10.1007/s12182-018-0243-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-018-0243-0