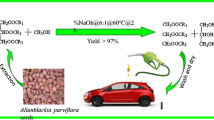
Abstract
In the present research work, a non-edible oil source Cucumis melo var. agrestis (wild melon) was systematically identified and studied for biodiesel production and its characterization. The extracted oil was 29.1% of total dry seed weight. The free fatty acid value of the oil was found to be 0.64%, and the single-step alkaline transesterification method was used for conversion of fatty acids into their respective methyl esters. The maximum conversion efficiency of fatty acids was obtained at 0.4 wt% NaOH (used as catalyst), 30% (methanol to oil, v/v) methanol amount, 60 °C reaction temperature, 600-rpm agitation rate and 60-min reaction time. Under these optimal conditions, the conversion efficiency of fatty acid was 92%. However, in the case of KOH as catalyst, the highest conversion (85%) of fatty acids was obtained at 40% methanol to oil ratio, 1.28 wt% KOH, 60 °C reaction temperature, 600-rpm agitation rate and 45 min of reaction time. Qualitatively, biodiesel was characterized through Fourier transform infrared spectroscopy (FTIR) and gas chromatography and mass spectroscopy (GC–MS). FTIR results demonstrated a strong peak at 1742 cm−1, showing carbonyl groups (C=O) of methyl esters. However, GC–MS results showed the presence of twelve methyl esters comprised of lauric acid, myristic acid, palmitic acid, non-decanoic acid, hexadecanoic acid, octadecadienoic acid and octadecynoic acid. The fuel properties were found to fall within the range recommended by the international biodiesel standard, i.e., American Society of Testing Materials (ASTM): flash point of 91 °C, density of 0.873 kg/L, viscosity of 5.35 cSt, pour point of − 13 °C, cloud point of − 10 °C, total acid number of 0.242 mg KOH/g and sulfur content of 0.0043 wt%. The present work concluded the potential of wild melon seed oil as excellent non-edible source of bioenergy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ecological balance of natural resources has been disrupted to alarming levels due to exponential growth of human population and many other anthropogenic activities (Eryilmaz and Yesilyurt 2016; Chang et al. 1996). The recent trends in urbanization and industrialization have not only increased fuel consumption but also led to depletion of finite fossil fuel resources (Ilkilic et al. 2011). The increased rate of fossil fuel consumption has resulted in increased carbon emissions, intensified local air pollution, ozone depletion and acid precipitation as well as magnified global warming up to alarming conditions (Ahmad et al. 2013). Therefore, the search for eco-friendly, renewable energy resources that may be substitutes for fossil fuels has become indispensable for a sustainable future. In the present scenario, biodiesel has a considerable attraction because of its many favorable properties: non-flammable, non-toxic, less pollutant emission, good lubricity and high flash point (Ahmad et al. 2011). Approximately, a 78% reduction in overall CO2 has been observed in various analyses of biodiesel because of its closed carbon cycle (Ahmad et al. 2011). In addition to the high biodegradability of biodiesel, its high rate of combustion efficiency and low viscosity (measure of resistance of fluid to gradual deformation by shear or tensile stress) are its other major advantages (Ahmad et al. 2007; Atabani et al. 2012). In many earlier studies, biodiesel had been synthesized from edible resources. Nevertheless, the use of edible oils causes insecurity of food supply (Ullah et al. 2015). To resolve this issue, the exploration of non-edible sources of seed oils for biodiesel synthesis has become indispensable (Demirbas 2003; Murugesan et al. 2009). Among accessible non-edible feedstocks, one of potential resources existing for biodiesel production may be Cucumis melo var. agrestis commonly known as wild melon, small gourd and wild musk melon across the world. Wild melon is an annual wild climber plant which grows up to 1.5 m. It is totally different from water melon with many distinct characteristics (Sahithi 2015). The stem is prostrate, branched covered with scabrous hair; calyx 1.5 mm long; corolla 6–8 mm long, yellow; flower solitary; fruit ellipsoid, round, with dark green stripes, 2.5–5 cm in diameter. It grows in a wide range of habitats, found especially in arid zones, but may grow in marsh landscapes, cypress heads, creek beds, sand bars, fallow fields, pastures, alfalfa, asparagus, rice, soybean and sugarcane fields, abandoned home sites, vacant lots, railroad banks, roadsides, dumps and medians, trash dumps and other disturbed areas (Nesom 2011). This plant species can be grown on waste and marshy lands where other crops do not grow easily. In fact, it is a non-edible wild plant mostly infesting pearl millet, sorghum, maize, cotton and range lands. However, in few areas of Pakistan, its fruit is consumed as a vegetable, but its seeds are totally non-edible. The seeds have considerable oil content which can be used for the biodiesel production. The fruit and seeds of wild melon are shown in Figs. 1 and 2, respectively.
To the best of our knowledge, a comprehensive literature review shows that no study has been done systematically on biodiesel production from Cucumis melo var. agrestis (wild melon). The aim and objectives of this study were to determine the oil and free fatty acid (FFA) content (%), to optimize various reaction variables of transesterification for maximum biodiesel yield, to characterize WMSO biodiesel by GC–MS and FTIR and to compare fuel properties of synthesized biodiesel by ASTM methods.
2 Materials and methods
The present research was conducted in the Biodiesel Laboratory, Department of Plant Sciences at Quaid-i-Azam University, Islamabad. Seeds of Cucumis melo var. agrestis were collected from wild habitats in Tehsil Talagang, Chakwal district, Punjab, Pakistan. Oil from the seeds of Cucumis melo var. agrestis (wild melon) was extracted mechanically using an electric oil expeller and tested via a chemical method using Soxhlet apparatus. The Soxhlet apparatus and oil cake are shown in Figs. 3 and 4, respectively.
The extracted WMSO was filtered, and FFA content was determined by aqueous acid–base titration. For this, two types of titration including blank and sample titration were carried out. For blank titration, 0.025 M KOH solution was prepared by dissolving 0.14 g KOH in 100 mL of distilled water, and the solution was loaded into a burette. Meanwhile, phenolphthalein indicator was prepared by mixing 0.5 g phenolphthalein in ethanol (50%). Blank titration was carried out using 10 mL of isopropyl alcohol in a conical flask and 2 or 3 drops of phenolphthalein indicator, while sample titration was carried out 1 mL of oil and 2–3 drops of phenolphthalein. In order to calculate accuracy or results, sample titration experiment was repeated twice and mean was calculated of the used volume of KOH. The free fatty acid number was calculated using readings of both titrations using the following formula.
where A is the volume used in sample titration, B the volume of KOH used in blank titration, C the amount of catalyst in g/L, D the volume of oil used in sample titration.
Based on the result of titrations, biodiesel was synthesized by a single-step method, i.e., alkali-catalyzed transesterification by the following methods applied by Kumar et al. (2015). During biodiesel synthesis from WMSO, methoxide was prepared by adding various concentrations of catalysts in methanol. Meanwhile, the oil was heated up to 120 °C and allowed to cool to 60 °C. At this temperature, methoxide was added to the oil. The reaction mixture was stirred for 45 min at 600 rpm. The product obtained was washed with distilled water resulting in a transparent reddish yellow product, much less viscous than WMSO, and it was considered as biodiesel by the following methods applied by Ullah et al. (2014). Various optimizations of WMSO were performed by changing one reaction variable while keeping all other operating parameters constant. Tested variables include the methanol to oil ratio, concentration of catalysts, reaction time and reaction temperature. During optimizations, the methanol to oil ratio was changed from 10% to 100% (v/v), the concentration of NaOH and KOH changed from 0 to 2.0% (w/w of oil taken), reaction temperature changed from 30 to 100 °C, and reaction time changed from 25 min to 2 h. Biodiesels in different optimization reactions are shown in Fig. 5.
For chemical characterization of biodiesel and confirmation of fatty acid methyl esters (FAMEs), GC–MS and FTIR analyses and determination of ASTM fuel properties were performed. For GC–MS, model GC-6860N directly coupled with mass spectrometer, model MS-597MSD was used. For FTIR spectroscopy, spectrometer Model Bruker Tensor 27 with a range of 4000–400 cm−1 was used. Studied fuel properties included color, density, flash point, pour point, kinematic viscosity at 40 °C, cloud point, sulfur contents tested by methods of ASTM.
3 Results and discussion
In the present study, the total content of oil in wild melon seeds was found to be approximately 29.1% and its total FFA was found to be 0.64 mg KOH/g. To ascertain the maximum biodiesel yield from WMSO, a range of transesterification reactions was performed using NaOH and KOH as catalyst. The effects of methanol to oil ratio, catalyst concentration, reaction time and temperature were studied in a series of reactions. The maximum yields of FAMEs and their optimized conditions are discussed below.
3.1 Effect of methanol to oil ratio on biodiesel yield
In order to achieve the maximum yield of biodiesel, various experiments were performed with the methanol to oil ratio varying from 10% to 100% (v/v), while other constant parameters were concentration of catalysts of 1.0 wt%, reaction time of 45 min, stirring speed of 600 rpm and reaction temperature of 60 °C. In optimization with KOH, the maximum yield (85%) of FAMEs was obtained with an optimum methanol to oil ratio of 40% (v/v), while in optimization with NaOH, a methanol to oil ratio of 30% (v/v) yielded the maximum FAMEs of 92. Beyond this limit, no significant effect of increasing the methanol to oil ratio was observed on methyl ester yield. However, further any increase in methanol to oil ratio resulted in difficulty in separation of two layers, i.e., biodiesel and glycerol. The results are shown in Fig. 6.
3.2 Effect of catalyst concentration on biodiesel yield
To optimize the concentration of catalysts, a number of reactions were performed with varying amounts of NaOH and KOH. For optimization with NaOH, 96% FAMEs yield was obtained by the addition of 0.4 wt% catalyst where other constant optimal conditions were as follows: methanol to oil ratio of 30% (v/v), reaction temperature of 60 °C, reaction time of 45 min. For optimizations with KOH, in series of reactions, maximum biodiesel yield (89%) was obtained at the addition of 1.28 wt% catalyst, while other constant reaction conditions were: methanol to oil ratio of 40% (v/v), reaction temperature of 60 °C, reaction time of 45 min. The former catalyst was found to be more favorable in the sense of quantified yield compared to the latter one. The effect of catalyst concentration is shown in Fig. 7.
3.3 Effect of reaction temperature on biodiesel yield
To study the effect of reaction temperature on biodiesel yield, a series of reactions were performed by keeping constant all other parameters while changing temperature during optimization reactions. In optimizations with NaOH, a maximum (96%) of biodiesel yield was obtained at 60 °C by keeping other conditions constant. The constant conditions were as follows: methanol to oil ratio of 30% (v/v), the catalyst concentration of 0.4 wt% and reaction time of 45 min. On the other hand, in optimization with KOH, optimized temperature was 60 °C for a maximum yield (89%) of biodiesel yield where constant conditions were: methanol to oil ratio of 40% (v/v), catalyst concentration of 1.28 wt% and reaction time of 45 min. During optimization of reaction temperature, a serious of reactions carried out showed that the reaction rate increased with the temperature to a certain extent. In results, the biodiesel yield increased proportionately with the increase in reaction temperature up to 60 °C. However, the yield of biodiesel decreased significantly at temperature higher than the boiling point of methanol (64.7 °C) due to emulsion formation. The effect of reaction temperature on biodiesel yield is shown in Fig. 8.
3.4 Effect of reaction time on biodiesel yield
To study the effect of time on transesterification reactions of WMSO, a series of reactions were performed with NaOH as well as KOH. With NaOH, the maximum yield of biodiesel was achieved at time of 60 min where other constant conditions were methanol (30% of oil), catalyst (0.4%) and temperature of 60 °C. Meanwhile, with KOH, the maximum yield of biodiesel was achieved at time of 45 min where other constant conditions were methanol (40% of oil), catalyst (1.28%) and reaction temperature of 60 °C. During both types of transesterification reactions, the minimum time required for initiation of biodiesel yield was 25 min, while below this no prominent phase separation was observed. Hence, the biodiesel yield increased proportionally from 25 to 60 min of time. In sense of time, KOH, as a catalyst, showed suitability over NaOH for biodiesel yield. The effect of reaction time on biodiesel synthesis from WMSO is shown in Fig. 9.
3.5 Chemical characterization by FTIR and GC–MS analysis
The synthesized biodiesel samples were characterized chemically by FTIR and GC–MS techniques, and results are shown in Figs. 10 and 11, respectively.
Fourier transform infrared spectroscopy (FTIR) is an analytical technique that is used to analyze various functional groups. In the present study, FTIR of biodiesel samples showed a strong peak at 1743 cm−1 that indicates the presence of methoxy ester carbonyl group. Based on the observed peaks, various functional groups found in FAMEs are shown in Table 1.
Gas chromatography and mass spectrometry (GC–MS) is used for the determination of fatty acids in a sample. The analyzed samples of biodiesel showed the diversity of fatty acids being converted into methyl esters. The GC–MS chromatogram of the synthesized biodiesel samples showed the presence of a total of twelve fatty acid methyl esters divided into saturated and unsaturated fatty acids. Among saturated fatty acids, dodecanoic acid, lauric acid (C12:0), methyl myristate and three hexadecanoic acid methyl esters including palmitic acid (C16:0) and methyl non-adecanoic acid were identified, while saturated fatty acids including two linoleic acid methyl esters that were 9, 12 octadecadienoic acid and one linoleic acid isopropyl ester were identified. Similarly, remaining methyl eicos-11-en-14-ynoate and 17-octadecynoic acid, methyl ester has unsatisfied carbon valences in their chemical composition as shown in Table 2.
3.6 Fuel properties
Fuel properties of biodiesel synthesized from WMSO were determined by the methods of American Society of Testing Materials (ASTM), and results are shown in Table 3. By the ASTM D-93 method, the flash point of WMSO biodiesel was found to be 91 °C. The higher flash point of biodiesel as compared to petro-diesel makes it safer to use, transport and store (Demirbaş 2006). The density of WMSO biodiesel was found to be 0.873 kg/L according to method ASTM D-1298. The density of biodiesel generally varies between strict limits of 0.86 and 0.90 g/cm3. Kinematic viscosity of WMSO biodiesel was determined to be 5.35 cSt at 40 °C, and it lies within the ASTM range of 1.9–6.0 cSt. The pour point or freezing point of biodiesel was tested to be − 13 °C according to ASTM D-97 method. The pour point of WMSO biodiesel is significantly lower than petro-diesel, so it is better in cold regions. Moreover, by the method ASTM D 6751, the pour point of WMSO biodiesel was in the range of − 15 to 16 °C. According to ASTM D-250 method, the cloud point of WMSO biodiesel was found to be − 10 °C. Biodiesel is considered to be environmentally biodegradable due to its negligible sulfur content. Sulfur content was measured and found to be 0.00431 wt% according to ASTM D-4294. Total acid number of the biodiesel was determined to be 0.242 mg KOH/g according to ASTM D-974. This value is much less than 0.8 and well within the range of standard ASTM D-6751, i.e., < 0.8. All the fuel properties of wild melon biodiesel met the international standards for biofuel.
4 Conclusion
Wild melon seeds were studied systematically for qualitative as well as quantitative biodiesel production using different transesterification reactions.
-
The total oil content in wild melon seeds was found to be 29.1% (w/w), while its free fatty acid (FFA) content found was 0.64%. Due to very low FFA content, the conversion of triglycerides was carried out by a single-step alkali-catalyzed transesterification.
-
In transesterification with NaOH, the highest conversion rate of WMSO into biodiesel was 96%. It was obtained using 30% methanol (v/v of oil), 0.4% catalyst (wt/wt of oil), at a reaction temperature 60 °C and reaction time of 1 h, whereas in transesterification with KOH, the maximum yield obtained was 89% where other optimized constant conditions were 40% methanol, 1.28 wt% catalyst, reaction temperature (60 °C) and 45 min of reaction time.
-
GC–MS-analyzed results indicated the presence of methyl esters including linoleic acid, palmitic acid, lauric acid, tetradecanoic acid, isopropyl linolate, octadecynoic acid. While methyl esters and octadecadienoic acid methyl ester were the main components found in biodiesel sample synthesized from WMSO, a strong FTIR peak at 1743 cm−1 confirmed the existence of a methoxy ester carbonyl group.
-
The fuel properties of synthesized FAMEs were in full agreement with ASTM standard D6751.
References
Ahmad A, Yasin NM, Derek C, Lim J. Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sustain Energy Rev. 2011;15(1):584–93. https://doi.org/10.1016/j.rser.2010.09.018.
Ahmad F, Khan AU, Yasar A. Transesterification of oil extracted from different species of algae for biodiesel production. Afr J Environ Sci Technol. 2013;7(6):358–64. https://doi.org/10.5897/AJEST12.167.
Ahmad S, Siwayanan P, Murad ZA, Aziz HA, Soi HS. Beyond biodiesel. Int News Fats Oils Related Mater. 2007;18(4):216–20.
Atabani AE, Silitonga AS, Badruddin IA, Mahlia T, Masjuki H, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev. 2012;16(4):2070–93. https://doi.org/10.1016/j.rser.2012.01.003.
Chang DY, Van Gerpen JH, Lee I, Johnson LA, Hammond EG, Marley SJ. Fuel properties and emissions of soybean oil esters as diesel fuel. J Am Oil Chem Soc. 1996;73(11):1549–55.
Demirbas A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey. Energy Convers Manag. 2003;44(13):2093–109. https://doi.org/10.1016/S0196-8904(02),00234-0.
Demirbas A. Oily products from mosses and algae via pyrolysis. Energy Sources Part A. 2006;28:933–40. https://doi.org/10.1080/009083190910389.
Eryilmaz T, Yesilyurt MK. Influence of blending ratio on the physicochemical properties of safflower oil methyl ester-safflower oil, safflower oil methyl ester-diesel and safflower oil-diesel. Renew Energy. 2016;95:233–47. https://doi.org/10.1016/j.renene.2016.04.009.
Ilkilic C, Aydın S, Behcet R, Aydin H. Biodiesel from safflower oil and its application in a diesel engine. Fuel Process Technol. 2011;92(3):356–62. https://doi.org/10.1016/j.fuproc.2010.09.028.
Kumar RS, Sureshkumar K, Velraj R. Optimization of biodiesel production from Manilkara zapota (L.) seed oil using Taguchi method. Fuel. 2015;140:90–6. https://doi.org/10.1016/j.fuel.2014.09.103.
Murugesan A, Umarani C, Chinnusamy TR, Krishnan M, Subramanian R, Neduzchezhain N. Production and analysis of bio-diesel from non-edible oils—a review. Renew Sustain Energy Rev. 2009;13(4):825–34. https://doi.org/10.1016/j.rser.2008.02.003.
Nesom G. New state records for Citrullus, Cucumis, and Cucurbita (Cucurbitaceae) outside of cultivation in the USA. Phytoneuron. 2011;1:1–7.
Sahithi GEA. Study of phytochemical and antioxidant activity of Cucumis melo var. agrestis fruit. J Pharmacogn Phytochem. 2015;4(2):303–6.
Ullah K, Ahmad M, Qureshi FA, Qamar R, Sharma VK, Sultana S, et al. Synthesis and characterization of biodiesel from Aamla oil: a promoting non-edible oil source for bioenergy industry. Fuel Process Technol. 2015;133:173–82. https://doi.org/10.1016/j.fuproc.2015.01.013.
Ullah K, Ahmad M, Sharma VK, Lu P, Harvey A, Zafar M, et al. Algal biomass as a global source of transport fuels: overview and development perspectives. Prog Nat Sci Mater Int. 2014;24(4):329–39. https://doi.org/10.1016/j.pnsc.2014.06.008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Xiu-Qin Zhu
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ameen, M., Zafar, M., Ahmad, M. et al. Wild melon: a novel non-edible feedstock for bioenergy. Pet. Sci. 15, 405–411 (2018). https://doi.org/10.1007/s12182-018-0227-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-018-0227-0