Abstract
A method for the simultaneous determination of caffeine and aspartame in various diet supplements and non-alcoholic beverages is presented. The analytes were analysed by high-performance liquid chromatography coupled to a charged aerosol detector (Corona CAD) and ultraviolet-diode array detector (UV-DAD) simultaneously connected in series. The method was validated using a Thermo Hypersil Gold-C18 column packed with 5 μm shell particles (150 × 4.6 mm) and acetonitrile–water (15/85% v/v) mobile phase at a flow rate of 1.00 mL/min. The elaborated method was validated for linearity, precision and accuracy. The rapid HPLC–CAD–UV-DAD technique is suitable for quantifying caffeine and aspartame in the range of 0.25–75 μg/mL in diet supplements and non-alcoholic beverages. The limit of detection for caffeine and aspartame was 62 and 43 ng/mL for Corona CAD and 31 and 30 ng/mL for UV-DAD detector, respectively. Each analyte calibration curve had a correlation coefficient of at least 0.999 and was linear in the defined range. The accuracies of CAD and UV-DAD detection were all acceptable, with the mean value of 100% for aspartame and 98.3% for caffeine. Precision values ranged from 0.09% to 1.12%. The work has demonstrated that charged aerosol detector can be successfully used in a rapid screening technique for biologically active substances in non-alcoholic beverages and diet supplements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food additives are strongly required by the modern food technologies, which try to fulfill consumers’ demands for variety, choice and convenience. These substances are used in food to maintain its nutritive quality, enhance its keeping quality and make it attractive or to aid in its processing, packaging or storage. Among them, caffeine and aspartame are widely used throughout the world.
Caffeine is one of a group of stimulants called methylxanthine or xanthine that occurs naturally in some plants. Products containing caffeine such as coffee, tea, soft drinks and chocolate are used and enjoyed by many people throughout the world. Besides, natural occurrence, caffeine is also used as a food additive regulated by the Federal Food and Drug Administration. Food and beverage manufacturers are legally obliged to list caffeine on their products’ label only when caffeine has been added. However, when caffeine is naturally present in other ingredients used in the production process need not be on the label. Caffeine can also be found listed as an active ingredient in some stimulants, cold remedies and various pain killers (Pawełczyk 1986).
This methylxanthine exhibits a number of biological effects resulting from its diuretic and stimulant properties. Some sensitive individuals experience side effects such as insomnia, headaches, irritability and nervousness when consuming caffeine; therefore, it is recommended to limit caffeine consumption (Pohler 2010; Wierzejska and Jarosz 2003).
Aspartame (l-alpha-aspartyl-l-phenylalanine methyl ester) is a low-calorie sweetener used to sweeten a wide variety of low-calorie foods and reduce calorie foods and beverages, including low-calorie tabletop sweeteners. Upon digestion, aspartame breaks down into three components (aspartic acid, phenylalanine and methanol), which are then absorbed into the blood and used in normal body processes. Neither aspartame nor its components accumulate in the body. These components are used in the body in the same ways as when they are derived from common foods (Butchko et al. 2002; Magnuson et al. 2007).
Although aspartame can be safely consumed by most healthy individuals, it has long been recognised that excessive intake of phenylalanine, one of the constituent amino acids of aspartame, can pose a hazard to individuals suffering from an inherited metabolic disorder called phenylketonuria (Butchko et al. 2002; Magnuson et al. 2007). For this reason, all foods containing aspartame must be indicated on the label for the presence of phenylalanine.
Currently, aspartame is consumed by over 200 million people around the world and is found in more than 6,000 products including carbonated soft drinks, powdered soft drinks, chewing gum, confections, gelatins, dessert mixes, puddings and fillings, frozen desserts, yogurt, tabletop sweeteners and some pharmaceuticals such as vitamins and sugar-free cough drops (European Parliament and Council Directive 94/35/EC 2011). The Food and Drug Administration, the Joint Expert Committee on Food Additives (JECFA) of the World Health Organization, the Scientific Committee for Food of the European Community and regulatory agencies in more than 100 countries have reviewed aspartame and found it safe for use (Butchko et al. 2002; Magnuson et al. 2007).
Low-calorie sweeteners provide consumers with many benefits, both psychological and physiological. Health professionals and consumers believe low-calorie sweeteners are effective for the following purposes: weight maintenance, weight reduction, management of diabetes, reduction of dental caries and reduction in the risks associated with obesity (Butchko et al. 2002).
High-performance liquid chromatography (HPLC) has been the most popular choice for the determination of additives (Wasik et al. 2007; Zygler et al. 2011; 2009); however, other analytical methods have also been applied in their analysis (Bergamo et al. 2011; Chen and Wang 2001; Zhu et al. 2005) but the method using HPLC–Corona charged aerosol detector (CAD) has not been reported up-to-date. The Corona CAD is a new type of detector introduced for HPLC applications and has recently become widely used (Błażewicz et al. 2010; Eom et al. 2010; Kou et al. 2009; Lísa et al. 2007; Nováková et al. 2009; Schönherr et al. 2009; Takahashi et al. 2008; Vervoort et al. 2008).
The detector Corona CAD measures a physical property of analyte and responds to almost all non-volatile species, independently of their nature and spectral or physico-chemical properties. The eluent from the HPLC column is nebulized with nitrogen and the droplets are dried to remove the mobile phase, producing analyte particles. A secondary stream of nitrogen becomes positively charged as it passes a high-voltage corona wire. This charge transfers difusionally to the opposing stream of analyte particles. The amount of charge acquired is directly proportional to particle size. The charge is then transferred to a collector where it is measured by a highly sensitive electrometer, generating a signal in direct proportion to the quantity of analyte present (Corona CAD 2011). It has been reported that Corona CAD can provide greater sensitivity, wider linear response range and better precision than other aerosol-based detectors such as ELSD (Eom et al. 2010; Takahashi et al. 2008; Vervoort et al. 2008). Moreover, Corona CAD is quite user-friendly since it does not require any optimization of operating parameters; therefore, it can be operated by any chromatographer without significant additional training (Schönherr et al. 2009; Corona CAD 2011).
The purpose of this work was to propose a new methodology for simultaneous determination of aspartame and caffeine by Corona CAD and ultraviolet-diode array detector (UV-DAD). This new method could be applied to the analysis of these two substances in food products and pharmaceutical preparations.
Experimental
Instrumentation
All experiments were performed on an HPLC Ultimate 3000 system (Dionex, Germering, Germany) consisting of: a quaternary pump, an autosampler and a column heater. A Corona CAD instrument (ESA, Chelmsford, MA, USA) was placed in series after the UV/DAD detector (Ultimate 3000, Dionex). Data processing was carried out with Chromeleon 6.8 software (Dionex). Nitrogen gas flow rate was regulated automatically and monitored by the CAD device and it was supplied by nitrogen generator Sirocco-5 (Schmidlin-DBS, Switzerland), regulated at 35 psi. Response range was set to 20 pA full scale. Medium filter was applied.
Chromatographic analysis was carried out at 30 °C. Samples were separated on a Thermo Hypersil (Thermo Fisher Scientific, Waltham, MA, USA) C18 column packed with 5 μm shell particles (150 × 4.6 mm) and acetonitrile–water (15/85% v/v) mobile phase at a flow rate of 1.00 mL/min. Particular chromatographic conditions are presented in Table 1. UV signal at 210 nm was recorded on the DAD using an acquisition rate of 10 points per second (Hertz). Total time of analysis amounted to 10 min.
Chemicals, Reagents and Standards
The chemicals used were of analytical reagent grade and all aqueous solutions were prepared using with ultra pure water (18.2 MΩ/cm) from a Milli-Q system (Millipore, MA, USA). HPLC-grade acetonitrile was obtained from J.T. Baker (Malinckrodt Baker B.V., Holland). Aspartame was obtained from Supelco (Bellefonte, USA) and caffeine from Fluka Analytical (China). Standards were made by dissolving 0.025 g of each individual analyte in an ultra pure water (aspartame) or methanol/water (50/50 v/v) mixture in separate 100-mL volumetric flasks. These solutions were further successively diluted with ultra pure water to obtain the required concentrations. All solutions were stored in a cool, dark place when not in use. The samples were stored for no more than 14 days.
Sample Preparation
Nineteen commercially available non-alcoholic beverages, including nine energy drinks, ten soft drinks and eight diet supplements and a tabletop sweetener were randomly obtained from the local market in Gdansk (Poland). The soft and energy drinks codes were A–E and F–E**, respectively. The letters signify different brands of products, whereas the asterisks mark different kinds of products of the same brand. In general, the purchased products represent a variety of beverages which contain caffeine and/or aspartame. Detailed characteristics of the analysed samples are presented in Table 2. Each of the 114 analytical subsamples analysed were injected in triplicate.
Samples of non-carbonated beverages were diluted, filtered and applied directly. The carbonated drink samples were degassed in a laboratory shaker bath for 90 min (in room temperature) and diluted with ultra-pure water as required. A 1-mL volume of cola drinks was directly diluted by deionized water to a 25-mL volumetric flask, whereas other products were diluted tenfold before HPLC analysis. In case of solid samples, an accurately weighed amount of 1 g of finely ground and homogenised diet supplements and tabletop sweetener was dissolved in ultra pure water in 100-mL volumetric flask. Solid samples solutions were also diluted tenfold before HPLC analysis. All the final solutions were filtered through 0.45-μm filters prior to analysis.
Validation of the Method
The quantitative analysis was done by HPLC with analysis conditions mentioned above. The data concerning method validation are summarised in Table 3.
The linearity of the detector response was determined by the square correlation coefficients of the calibration curves generated by six repeated injections of standard solutions at 12 concentration levels in the range of 0.15–75 μg/mL or 0.25–75 μg/mL (Table 3). The calibration curves were obtained by plotting concentration (in micrograms per milliliter) against peak area. Although it is known that Corona CAD response is nonlinear at a range of four orders of magnitude, its signal is nearly linear in the smaller ranges (Błażewicz et al. 2010; Corona CAD 2011). Responses obtained in the examined range (Table 3) were expressed by a linear equation y = ax + b with good r 2 determination coefficient values. The estimation of the detection and quantitation limits was done based on the calibration curve, using 3 SD/b and 10 SD/b (SD is the standard deviation of the curve and b is the slope of the curve) (Table 3).
Repeatability was assessed using three concentrations covering the specified range for the procedure. The precision was calculated from three consecutive injections for each concentration and the observed RSD ranged from 0.09% to 1.12% (Table 3). Intermediate precision was calculated for 2 days.
Recoveries were determined by standard addition method, i.e. by spiking a soft drink and a diet supplement with known amounts of aspartame and caffeine (Table 3). The accuracy of the recovery for aspartame and caffeine added to a soft drink and a diet supplement was evaluated at three levels, i.e. standard concentrations values were as follows: 35, 70 and 140 μg/mL for aspartame and 150, 300 and 600 μg/mL for caffeine. The mean recoveries for aspartame from each run were in the range of 99.8–103% for Corona CAD and 99.6–101% for UV-DAD, whereas for caffeine these, were between 97.5% and 98.8% for Corona CAD and 96.1–100.4% for UV-DAD.
Results and Discussion
In the current study, the identification and determination of aspartame and caffeine in diet supplements and non-alcoholic beverages by means of HPLC–UV-DAD and HPLC–Corona CAD are presented. The analytical methodology revealed good linearity, sensitivity, exactitude and precision (Table 3). All samples were analysed according to the experimental conditions (Table 1). The results concerning diet supplements are summarised in Table 4 and non-alcoholic beverages in Table 5. The aspartame and caffeine concentrations in the samples are characterised by arithmetic mean value for wet weight basis and the corresponding SD.
The results demonstrated in Tables 4 and 5 reveal a good agreement between those determined by these two methods, which indicates that Corona CAD would be a beneficial alternative to UV-DAD for the separation and determination of these substances. The average aspartame concentration in diet supplements was in the range of 1.93 and 27.7 mg per tablet (sachet) (Table 4). The lowest amount was determined in the diet supplement recommended for children over 4 years old (product T), whereas the highest in the product designed for students and people having problems with concentration and memory (product L) (Table 4). Average aspartame concentration in tabletop sweetener was determined between 16.6 mg (Corona CAD) and 19.4 mg/tablet/sachet (UV-DAD). Among soft drinks, the highest average aspartame concentration was found in a product D (cola drink), i.e. 58.6 mg/100 mL (UV-DAD) or 53.3 mg/100 mL (Corona CAD). Sugar-free energy drinks contained aspartame between 7.12 and 10 mg/100 mL (UV-DAD) or 5.92–9.21 mg/100 mL (Corona CAD). According to Majewska (2008), aspartame content in two types of tabletop sweeteners was in the range of 172 and 374 mg/g, which is comparable to our results. However, Cantarelli et al. (2009) determined much lower levels of this additive in tabletop sweeteners. Sękalska (2007) determined aspartame in a popular diet cola drink, i.e. 58.6 mg/100 mL. Similar levels of aspartame in soft drinks were found by Wróbel and Wróbel (1997) and Gibbs et al. (1996).
The acceptable daily intake (ADI) value for aspartame determined by JECFA is 0–40 mg/kg body mass (up to 2,800 mg/day for 70 kg man/woman) (Aspartame 2011). Maximum daily intake of aspartame with the analysed supplements is much lower than the ADI, and therefore, the exposure to exceed the acceptable daily intake of this sweetener is very small. The greatest value of the exposure (2.5%) was recorded in the case of a product “O”, which according to the manufacturer can be consumed up to three times a day. On the other hand, the exposure through consumption of the product “L” (dosage—two times per day), which contained the highest concentration of aspartame, amounted to 1.98%. Consumption of 250 mL of energy drinks on average supplies human organism with 0.78% of ADI; however, consumption of 250 mL of product “D” gives 5.5% of ADI dose for aspartame.
Caffeine was found in all the analysed soft and energy drinks (Table 5). The highest caffeine concentration, 163 mg/100 mL (UV-DAD), was determined in an energy drink (product K)—one of the newest products on Polish market that constitutes a condensed form of conventional energy drinks. Other energy drinks contained this substance at the level oscillating around 30 mg/100 mL, whereas cola drinks around 10 mg/100 mL. The lowest amount of caffeine was found in soft drink “B”, 3.87 mg/100 mL (Corona CAD). The results concerning caffeine presented in this study are generally comparable to the data reported by Armenta et al. (2005), Lucena et al. (2005) and Aranda and Morlock (2000).
In assessing consumer exposure to caffeine, a specific reference value should be used, i.e. a maximum daily dose for an adult set by Polish Pharmacopoeia, which is amounting to 1.5 g (Pharmacopeia 2008). The assessment of exposure to caffeine in soft drinks has shown that the consumption of 250 mL of the analysed products resulted in the greatest exposure in case of the product “K” (27%) and lowest in the case of the product “B” (0.71%). This evaluation is very important as there are cases of fatal caffeine intoxication (Holmgren et al. 2004; Kerrigan and Lindsey 2005). However, susceptibility to poisoning through exposure to caffeine cannot be unambiguously determined due to very large individual variation, age, type of ingested diet, medications or even smoking.
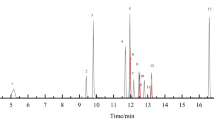
Figure 1a, b illustrates the results of HPLC analysis with UV-DAD and Corona CAD detection, respectively, for the exemplary samples of energy drinks. As can be observed, aspartame and caffeine could be determined in two cases, samples “H” and “J”, which is in agreement with the producer declaration on the label, and in two samples “F” and “G”, in which only caffeine was found. Therefore, it can be concluded that this method is able to determine simultaneously aspartame and caffeine in real food samples so it can be used for the routine analysis in control laboratories. What’s more, in each of the samples analysed which have been declared to contain aspartame and caffeine, these substances were determined.
Conclusions
The HPLC–Corona CAD method with UV detection offers high accuracy, good repeatability and reproducibility and a relative short analysis time for quantification of aspartame and caffeine in food samples. The simple mobile phase and the isocratic elution yielded low detection limits and good resolution within a minimum analysis time of 10 min. As the results of real sample analysis are very satisfactory, this method can be used for the routine analysis of aspartame and caffeine in drinks and diet supplements. The development of better tools for analysis of bioactive constituents in food products that could be hazardous to human health will continue to be important because all such activities will ultimately contribute to the overall quality and safety of food.
References
Aranda M, Morlock G (2000) J Chrom A 1131:153
Armenta S, Garrigues S, Guardia M (2005) Anal Chim Acta 547:197
Aspartame (2011) INCHEM. http://www.inchem.org/documents/jecfa/jecmono/v16je03.htm. Accessed 3 Mar 2011
Bergamo AB, da Silva JAF, de Jesus DP (2011) Food Chem 124:1714
Błażewicz A, Fijałek Z, Warowna-Grześkiewicz M, Jadach M (2010) J Chrom A 1217:1266
Butchko HH, Stargel WW, Comer CP et al (2002) Regul Toxicol Pharmacol 35:S1
Cantarelli MA, Pellerano RG, Marchevsky EJ, Camiña JM (2009) Food Chem 115:1128
Chen QC, Wang J (2001) J Chrom A 937:57
Corona CAD (2011) Polygen information sheet. http://www.polygen.com.pl/presentations/corona_cad.pdf. Accessed 5 Mar 2011
Eom HY, Park S-Y, Kim MK et al (2010) J Chrom A 1217:4347
European Parliament and Council Directive 94/35/EC of 30 June 1994 on sweeteners intended for use in foodstuffs (2011). Accessed 6 Apr 2011
Gibbs BF, Alli I, Mulligan CN (1996) J Chrom A 725:372
Holmgren P, Norden-Pettersson L, Ahlner J (2004) Foren Sci Intern 139:71
Kerrigan S, Lindsey T (2005) Foren Sci Intern 153:67
Kou D, Manius G, Zhan S, Chokshi HP (2009) J Chrom A 1216:5424
Lísa M, Lynen F, Holčapek M, Sandra P (2007) J Chrom A 1176:135
Lucena R, Cardenas S, Gallego M, Valcarcel M (2005) Anal Chim Acta 530:283
Magnuson BA, Burdock GA, Doull J et al (2007) Crit Rev Toxicol 37:629
Majewska E (2008) Żywność 2:106
Nováková L, Solichová D, Solich P (2009) J Chrom A 1216:4574
Pawełczyk E (1986) Chemia leków. PZWL, Warszawa (in Polish)
Farmakopea Polska VIII (Polish Pharmacopeia) (2008) Polskie Towarzystwo Farmaceutyczne, Warszawa, 3276 (in Polish)
Pohler H (2010) J Nurse Pract 1:49
Schönherr C, Touchene S, Wilser G, Peschka-Süss R, Francese G (2009) J Chrom A 1216:781
Sękalska B (2007) Żywność 3:127
Takahashi K, Shinichi K, Senda M et al (2008) J Chrom A 1193:151
Vervoort N, Daemen D, Tӧrӧk G (2008) J Chrom A 1189:92
Wasik A, McCourt J, Buchgraber M (2007) J Chrom A 1157:187
Wierzejska R, Jarosz M (2003) Żyw Człow Metab 30:1234
Wróbel K, Wróbel K (1997) J Chrom A 733:163
Zhu Y, Guo Y, Ye M, James FS (2005) J Chrom A 1085:143
Zygler A, Wasik A, Namieśnik J (2009) Tr Anal Chem 28:1082
Zygler A, Wasik A, Kot-Wasik A, Namieśnik J (2011) Anal Bioanal Chem 400:2159
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Grembecka, M., Szefer, P. Simultaneous Determination of Caffeine and Aspartame in Diet Supplements and Non-Alcoholic Beverages Using Liquid-Chromatography Coupled to Corona CAD and UV-DAD Detectors. Food Anal. Methods 5, 1010–1017 (2012). https://doi.org/10.1007/s12161-011-9334-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9334-x