Abstract
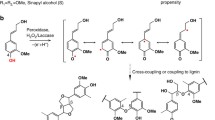
Partial substitution of normal monolignols with phenolic precursors from other metabolic pathways may improve the susceptibility of lignified biomass to chemical pretreatment and enzymatic saccharification for biofuel production. Flavonoids and gallate esters readily undergo oxidative coupling reactions, suggesting they could serve as alternate monomers for forming lignin in plants. To test this premise, primary cell walls of Zea mays (L.) were artificially lignified with normal monolignols plus various flavan-3-ol/phenolic ester derivatives, flavonol glycoside/gallate ester derivatives, or pentagalloyl glucose added as 0 or 45 % of the precursor mixture. Most alternate monomers readily copolymerized with normal monolignols, but wall-bound lignin was most efficiently formed with epicatechin, epicatechin gallate, epigallocatechin gallate, or hyperoside. Yields of glucose from a high-throughput digestibility platform were used to examine how lignin modifications affected the susceptibility of cell walls to enzymatic hydrolysis following alkaline or acidic pretreatments of different severities. With the exception of hyperoside, incorporation of alternate monomers into lignin improved yields of enzymatically released glucose by 18–60 % after mild alkaline pretreatment and by 6–34 % after mild acid pretreatment. Responses due to lignin modification diminished as pretreatment severity increased. Overall, our results suggest that apoplastic deposition of pentagalloyl glucose or gallated flavan-3-ols such as epicatechin gallate or epigallocatechin gallate for incorporation into lignin could be promising plant genetic engineering targets for improving sugar yields from grass biomass crops that are subjected to low-temperature alkaline pretreatments.


Similar content being viewed by others
References
Dale BE, Ong RG (2014) Design, implementation, and evaluation of sustainable bioenergy production systems. Biofuels Bioprod Bioref 8:487–503
Kim TH, Kim TH (2014) Overview of technical barriers and implementation of cellulosic ethanol in the U.S. Energy 66:13–19
Zeng Y, Zhao S, Yang S, Ding S-Y (2014) Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27:38–45
Xu Z, Huang F (2014) Pretreatment methods for bioethanol production. Appl Biochem Biotechnol 174:43–62
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761
van der Weijde T, Kamei CLA, Torres AF, Vermerris W, Dolstra O, Visser RGF, Trindade LM (2013) The potential of C4 grasses for cellulosic biofuel production. Front Plant Sci 4:107
Tang W, Tang AY (2014) Transgenic woody plants for biofuel. J For Res 25:225–236
Casler MD, Jung HJG (2006) Relationships of fibre, lignin, and phenolics to in vitro fibre digestibility in three perennial grasses. Anim Feed Sci Technol 125:151–161
Pilate G, Dejardin A, Leple JC (2012) Field trials with lignin-modified transgenic trees. In: Jouanin L, Lapierre C (eds) Adv Bot Res, Vol 61 Elsevier Ltd, pp 2–36
Jung HJG, Samac DA, Sarath G (2012) Modifying crops to increase cell wall digestibility. Plant Sci 185–186:65–77
Sederoff RR, MacKay JJ, Ralph J, Hatfield RD (1999) Unexpected variation in lignin. Curr Opin Plant Biol 2:145–152
Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, Boerjan W (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem Rev 3:29–60
Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, Boerjan W (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196:978–1000
Ralph J (2006) What makes a good monolignol substitute? In: Hayashi T (ed) The science and lore of the plant cell wall. Biosynthesis, structure and function. BrownWalker Press, Boca Raton, pp 285–293
Grabber JH, Schatz PF, Kim H, Lu F, Ralph J (2010) Identifying new lignin bioengineering targets: 1. Monolignol-substitute impacts on lignin formation and cell wall fermentability. BMC Plant Biol 10:114
Grabber JH, Ralph J, Hatfield RD, Quideau S, Kuster T, Pell AN (1996) Dehydrogenation polymer-cell wall complexes as a model for lignified grass walls. J Agric Food Chem 44:1453–1459
Grabber JH (2005) How do lignin composition, structure, and cross-linking affect degradability? a review of cell wall model studies. Crop Sci 45:820–831
Grabber JH, Hatfield RD, Lu F, Ralph J (2008) Coniferyl ferulate incorporation into lignin enhances the alkaline delignification and enzymatic degradation of cell walls. Biomacromolecules 9:2510–2516
Wilkerson CG, Mansfield SD, Lu F, Withers S, Park J-Y, Karlen SD, Gonzales-Vigil E, Padmakshan D, Unda F, Renecoret J, Ralph J (2014) Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344:90–93
Grabber JH, Ress D, Ralph J (2012) Identifying new lignin bioengineering targets: impact of epicatechin, quercetin glycoside, and gallate derivatives on the lignification and fermentation of maize cell walls. J Agric Food Chem 60:5152–5160
Elumalai S, Tobimatsu Y, Grabber JH, Pan X, Ralph J (2012) Epigallocatechin gallate incorporation into lignin enhances the alkaline delignification and enzymatic saccharification of cell walls. Biotechnol Biofuels 5:59
Tobimatsu Y, Elumalai S, Grabber JH, Davidson CL, Pan X, Ralph J (2012) Hydroxycinnamate conjugates as potential monolignol replacements: in vitro lignification and cell wall studies with rosmarinic acid. ChemSusChem 5:676–686
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Lu F, Ralph J (2008) Novel tetrahydrofuran structures derived from β − β-coupling reactions involving sinapyl acetate in kenaf lignins. Org Biomol Chem 6:3681–3694
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Hatfield RD, Jung HG, Ralph J, Buxton DR, Weimer PJ (1994) A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J Sci Food Agric 65:51–58
Foster CE, Martin TM, Pauly M (2010) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) Part I: Lignin. http://www.jove.com/details.php?id=1745
Foster CE, Martin TM, Pauly M (2010) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) Part II: Carbohydrates. http://www.jove.com/details.php?id=1837
Santoro N, Cantu SL, Tornqvist CE, Falbel TG, Bolivar JL, Patterson SE, Pauly M, Walton JD (2010) A high-throughput platform for screening milligram quantities of plant biomass for lignocellulose digestibility. Bioenergy Res 3:93–102
Grabber JH, Ralph J, Hatfield RD (1998) Ferulate cross-links limit the enzymatic degradation of synthetically lignified primary walls of maize. J Agric Food Chem 46:2609–2614
Grabber JH, Hatfield RD, Ralph J (1998) Diferulate cross-links impede the enzymatic degradation of nonlignified maize walls. J Sci Food Agric 77:193–200
SAS (2010) PC windows version 9.2. SAS Institute Inc, Cary, NC
Saxton AM (1998) A macro for converting mean separation output to letter groupings in proc mixed. Proc 23rd SAS Users Group Intl, SAS Institute, Cary NC, pp 1243–1246
Piepho HP, Williams ER, Fleck M (2006) A note on the analysis of designed experiments with complex treatment structure. Hortscience 41:446–452
Darvill AG, Smith CJ, Hall MA (1978) Cell wall structure and elongation growth in Zea mays coleoptile tissue. New Phytol 80:503–516
Carpita NC (1984) Cell wall development in maize coleoptiles. Plant Physiol 76:205–212
Hatfield RD, Grabber JH, Ralph J, Brei K (1999) Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J Agric Food Chem 47:628–632
Yasuda S, Fukushima K, Kakehi A (2001) Formation and chemical structures of acid-soluble lignin I: sulfuric acid treatment time and acid-soluble lignin content of hardwood. J Wood Sci 47:69–72
Acknowledgments
This work was funded by Stanford University’s Global Climate and Energy Project (GCEP) and by USDA-ARS in-house funds. CF, NS, and JR were funded by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494). The authors thank Novozymes (Franklinton, NC) for generously providing enzymes for this research. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grabber, J.H., Santoro, N., Foster, C.E. et al. Incorporation of Flavonoid Derivatives or Pentagalloyl Glucose into Lignin Enhances Cell Wall Saccharification Following Mild Alkaline or Acidic Pretreatments. Bioenerg. Res. 8, 1391–1400 (2015). https://doi.org/10.1007/s12155-015-9605-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9605-2