Abstract
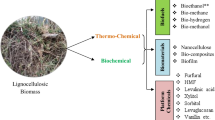
Fast-growing hybrid poplar species are attractive as feedstocks for the production of bio-based chemicals including biofuels. Pretreatment makes cellulose accessible to enzymatic hydrolysis, producing hemicellulose and lignin as major co-products. Poplar woodchips pretreated hydrothermally in an experimental industrial pilot-plant generated two prehydrolyzate streams; purge-condensate collected from cooking the woodchips and press-filtrate collected after the fiber explosion step. Both prehydrolyzates contain hemicellulose sugars (pentoses, hexoses, oligosaccharides), acetic acid, and degradation products from hemicellulose and lignin (furanic and phenolic compounds, respectively). Purge prehydrolyzate was highly diluted, of low sugar content and high biochemical/chemical oxygen demand, and constituted a process waste that was addressed for value-adding through production of enzymes by fermentation. Wood-decay fungi were screened for laccases on purge prehydrolyzate, with Botryosphaeria rhodina producing highest titers. B. rhodina detoxified the prehydrolyzate but at expense of sugar consumption. Adding xylose and glycerol to purge did not inhibit fungal growth or impede laccase production. Phenolics in the purge had inducing effect on enhancing laccase titers. Laccase production was optimized by Box-Behnken-(33)-factorial design varying the concentration of xylose in the purge, together with adding copper as laccase inducer and glycerol to increase the level of fermentable substrate, resulting in optimal enzyme titers (36.37 ± 3.52U/mL; validated). The crude laccase preparation was employed to detoxify the more concentrated press prehydrolyzate, reducing the overall content of phenolics by ∼30 %. Chromatographic (high-performance liquid chromatography-ultraviolet, gas chromatography/mass spectrometry) analysis identified various phenolic compounds present in the press prehydrolyzate following laccase treatment, and in the presence of the mediator, ABTS, the phenolics content decreased further.




Similar content being viewed by others
References
Sannigrahi P, Ragauskas AJ, Tuskan GA (2010) Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels Bioprod Bioref 4:209–226
Taherzadeh MJ, Karimi K (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources 2:472–499
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Zhang X, Tu M, Paice MG (2011) Routes to potential bioproducts from lignocellulosic biomass, lignin and hemicelluloses. Bioenerg Res 4:246–257
Werpy T, Petersen G (2004) Top value added chemicals from biomass. Volume I: results of screening for potential candidates from sugars and synthesis gas. Pacific Northwest National Laboratory, National Renewable Energy Laboratory, Office of Biomass Program, U.S. Department of Energy (DOE), Oak Ridge, TN (USA)
Wen S, Liu L, Nie KL, Deng L, Tan TW, Fang W (2013) Enhanced fumaric acid production by fermentation of xylose using a modified strain of Rhizopus arrhizus. Bioresources 8:2186–2194
Massen N, Panakova M, Wierckx N, Geiser E, Zimmermann M, Bölker M, Klinner U, Blank LM (2014) Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng Life Sci 14:129–134
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol Biofuels 6:15
Clark TA, Mackie KL (1984) Fermentation inhibitors in wood hydrolysates derived from the softwood Pinus radiata. J Chem Technol Biotechnol 34:101–110
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16
Messerschmidt A (1997) Multi-copper oxidases. World Scientific, Singapore
Arora DS, Sharama RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788
Madhavi V, Lele SS (2009) Laccase: properties and applications. Bioresources 4:1694–1717
Giese EC, Dekker RFH, Barbosa AM (2008) Orange baggase as substrate for the production of pectinase and laccase by Botryosphaeria rhodina MAMB-05 in submerged and solid state fermentation. Bioresources 3:335–345
Gómez J, Pazos M, Couto SR, Sanromán MA (2005) Chestnut shell and barley bran as potential substrates for laccase production by Coriolopsis rigida under solid-state conditions. J Food Eng 68:315–319
Meza JC, Auria R, Lomascolo A, Sigoillot J-C, Casalot L (2007) Role of ethanol on growth, laccase production and protease activity in Pycnoporus cinnabarinus ss3. Enzym Microb Technol 41:162–168
Dekker RFH, Barbosa AM, Sargent K (2002) The effect of lignin-related compounds on the growth and production of laccases by the ascomycete, Botryosphaeria sp. Enzym Microb Technol 30:374–380
Dekker RFH, Barbosa AM, Giese EC, Godoy SDS, Covizzi LG (2007) Influence of nutrients on enhancing laccase production by Botryosphaeria rhodina MAMB-05. Int Microbiol 10:177–185
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of ash in biomass. Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510-42622. National Renewable Energy Laboratory, Golden, Colorado
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510-42618. National Renewable Energy Laboratory, Golden, Colorado
Barbosa AM, Dekker RFH, ST. Hardy GE (1996) Veratryl alcohol as an inducer of laccase by an ascomycete, Botryosphaeria sp., when screened on the polymeric dye, poly R-478. Lett Appl Microbiol 23:93–96
Vogel HJ (1956) A convenient growth medium for Neurospora crassa (medium N). Microbial Genet Bull 13:42–43
Hill T, Lewicki P (2006) Experimental design (industrial DOE). In: Statistics: methods and applications: a comprehensive reference for science, industry, and data mining, 1st edn. StatSoft Inc., Tulsa, OK, USA, pp 179–226. ISBN 1-884233-59-7
STATISTICA (2009) StatSoft Inc. http://www.statsoft.com
Kiefer K, Herwehe K (1996) Use of BSTFA silylating reagent to prepare volatile derivatives for GC. Report SUPELCO 15(4):1–5
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Amer J Enol Viticult 16:144–153
APHA-AWWA-WEF (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA)-American Water Works Association (AWWA)-Water Environment Federation (WEF): Washington, DC, USA pp. 1496
US-EPA (Environmental Protection Agency) Method 410.4 (1993) The determination of chemical oxygen demand by semi-automated colorimetry. J. W. O’Dell, Editor, Inorganic Chemistry Branch, Chemistry Research Division, Revision 2.0 http://www.caslab.com/EPA-Methods/PDF/EPA-Method-4104.pdf
Verardo V, Arráez-Román D, Segura-Carretero A, Marconi E, Fernández-Gutiérrez A, Caboni MF (2011) Determination of free and bound phenolic compounds in buckwheat spaghetti by RP-HPLC-ESI-TOF-MS: effect of thermal processing from farm to fork. J Agric Food Chem 59:7700–7707
Matheson CD, McCollum AJ (2014) Characterising native plant resins from Australian Aboriginal artefacts using ATR-FTIR and GC/MS. J Archaeol Sci 52:116–128
Salvador LD, Suganuma T, Kitahara K, Tanoue H, Ichiki M (2000) Monosaccharide composition of sweet potato fiber and cell wall polysaccharides from sweet potato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J Agric Food Chem 48:3448–3454
Vasconcelos de Sá LR, de Oliveira MAL, Cammarota MC, Matos A, Ferreira-Leitão (2011) Simultaneous analysis of carbohydrates and volatile fatty acids by HPLC for monitoring fermentative biohydrogen production. Int J Hydrogen Energy 1–10
US-EPA Method 3051A (2007), Microwave assisted acid digestion of sediments, sludges, soils and oils. In: Test methods for evaluating solid waste. US Environmental Protection Agency, Washington, DC, USA, 3rd edition
Rao KS, Balaji T, Rao TP, Babu Y, Naidu GRK (2002) Determination of iron, cobalt, nickel, manganese, zinc, copper, cadmium and lead in human hair by inductively coupled plasma atomic emission spectrometry. Spectrochim Acta B 57:1333–1338
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol Biofuels 5:13
Saldanha RL, Garcia JE, Dekker RFH, Vilas-Boas LA, Barbosa AM (2007) Genetic diversity among Botryosphaeria isolates and their correlation with cell wall-lytic enzyme production. Braz J Microbiol 38:259–264
Scheller HV, Ulvskov P (2010) Hemicelluloses. Ann Rev Plant Biol 61:263–289
Deutschmann R, Dekker RFH (2012) From plant biomass to bio-based chemicals: latest developments in xylan research. Biotechnol Adv 30:1627–1640
Dekker RFH, Karageorge H, Wallis AFA (1987) Pretreatment of hardwood (Eucalyptus regnans) sawdust by autohydrolysis explosion and its saccharification by Trichodermal cellulases. Biocatal Biotransfor 1:47–61
Chua MGS, Wayman M (1979) Characterization of autohydrolysis aspen (Populus tremuloides) lignins. Part 1. Composition and molecular weight distribution of extracted autohydrolysis lignin. Can J Chem 57:1141–1149
Leschinsky M, Webber HK, Patt R, Sixta H (2009) Formation of insoluble components during autohydrolysis of Eucalyptus globulus. Lenzinger Berichte 87:16–25
Stewart JJ, Kadla JF, Mansfield SD (2006) The influence of lignin chemistry and ultrastructure on the pulping efficiency of clonal aspen (Populus tremuloides Michx.). Holzforschung 60:111–122
Vasconcelos AFD, Barbosa AM, Dekker RFH, Scarminio IS, Rezende MI (2000) Optimization of laccase production by Botryosphaeria sp. in the presence of veratryl alcohol by the response-surface method. Process Biochem 35:1131–1138
Dekker RFH, Barbosa AM (2001) The effects of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enz Microb Technol 28:81–88
Andrade MM, Barbosa AM, Bofinger MR, Dekker RFH, Messias JM, Guedes CL, Zaminelli T, de Oliveira BH, de Lima VM, Dall’Antonia LH (2013) Lipase production by Botryosphaeria ribis EC-01 on soybean and castorbean meals: optimization, immobilization, and application for biodiesel production. Appl Biochem Biotechnol 170:1792–1806
Couto SR, Rodríguez A, Paterson RRM, Lima N, Teixeira JA (2006) Laccase activity from the fungus Trametes hirsute using an air-lift bioreactor. Lett Appl Microbiol 42:612–616
Alves Da Cunha MA, Barbosa AM, Giese EC, Dekker RFH (2003) The effect of carbohydrate carbon sources on the production of constitutive and inducible laccases by Botryosphaeria sp. J Basic Microbiol 43:385–392
Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G (2000) Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol 2000:920–924
Galhaup HD (2001) Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol 56:225–232
Lacina C, Gourene G, Spiros AN (2003) Utilization of fungi for biotreatment of raw wastewaters. Afr J Biotechnol 2:620–630
Palmqvist E, Hahn-Hӓgerdal B, Szengyel Z, Zacchi G, Rèczey K (1997) Simultaneous detoxification and enzyme production of hemicellulose hydrolysates obtained after steam pretreatment. Enzym Microb Technol 20:286–293
Larsson S, Reimann A, Nilvebrant N-O, Jönsson LJ (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77:91–103
Nichols NN, Sharma LN, Mowery RA, Chambliss CK, Peter van Walsum G, Dien BS, Iten LB (2008) Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzym Microb Technol 42:624–630
Basha KM, Rajendran A, Thangavelu V (2010) Recent advances in the biodegradation of phenol: a review. Asian J Exp Biol Sci 1:219–234
Al-Khalid T, El-Naas MH (2012) Aerobic biodegradation of phenols: a comprehensive review. Crit Rev Environ Sci Technol 42:1631–1690
Ran H, Zhang J, Gao Q, Lin Z, Bao J (2014) Analysis of biodegradation performance of furfural and 5-hydroxymethylfurfural by Amorphotheca resinae ZN1. Biotechnol Biofuels 7:51
Jurado M, Prieto A, Martínez-Alcalá A, Martínez AT, Martínez MJ (2009) Laccase detoxification of steam-exploded wheat straw for second-generation bioethanol. Bioresour Technol 100:6378–6384
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31
Lee K-M, Kalyani D, Tiwari MK, Kim T-S, Dhiman SS, Lee J-K, Kim I-W (2012) Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Bioresour Technol 123:636–645
Moreno AD, Ibarra D, Fernández JL, Ballesteros M (2012) Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyvermoyces marxianus CECT 10875. Bioresour Technol 106:101–109
Alvira P, Moreno AD, Ibarra D, Sáez F, Ballesteros M (2013) Improving the fermentation performance of Saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol Prog 29:74–82
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane baggase hydrolysate improves ethanol production by Candida shehate NCIM 3501. Bioresour Technol, 1947–1950
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram U, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Desai SS, Nityanand C (2011) Microbial laccases and their applications: a review. Asian J Biotechnol 3:98–124
Acknowledgments
The authors gratefully acknowledge CRIBE (Center for Research and Innovation in the Bio-economy, Ontario, Canada) for a grant (RFH Dekker) that supported this work. Dr. Regis Benech, Dr. Stephan Brey, and Greg Santavy of Greenfield Engineering and Technology (Research and Development), Chatham (Canada), are thanked for providing the poplar prehydrolyzate samples. Dr. Rudi Deutschmann is acknowledged for providing data on the compositional analysis of the poplar woodchips. The authors gratefully acknowledge the technical expertise of Greg Kepka and Gamini Rupasingha for their assistance with the HPLC analyses.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vithanage, L.N.G., Barbosa, A.M., Borsato, D. et al. Value Adding of Poplar Hemicellulosic Prehydrolyzates: Laccase Production by Botryosphaeria rhodina MAMB-05 and Its Application in the Detoxification of Prehydrolyzates. Bioenerg. Res. 8, 657–674 (2015). https://doi.org/10.1007/s12155-014-9547-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-014-9547-0