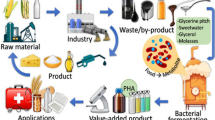
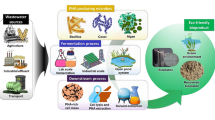
Abstract
Polyhydroxyalkanoates have attracted great interest as a suitable alternative to petrochemical based plastics due to their outstanding properties such as biodegradability and biocompatibility. However, the biggest problem in the production of microbial polyhydroxyalkanoates is low cost-effectiveness. In this study, polyhydroxyalkanoate production was carried out using waste substrates with local isolates. Culture conditions were optimized to increase the polyhydroxyalkanoate production potential. The produced polyhydroxyalkanoate was characterized by FTIR analyses, and its metabolic pathway was determined by real-time PCR. According to the results, the best polyhydroxyalkanoate producer bacteria was characterized as Pseudomonas neustonica NGB15. The optimal culture conditions were detected as 30 g/L banana peel powder, 25 °C temperature, pH 8, and 4-day incubation time. Under the optimized conditions, 3.34 g/L PHA production was achieved. As a result of FTIR analyses, major peaks were obtained at 1723, 1277, 1261, 1097, 1054, and 993 cm−1. These peaks represent that the type of produced polyhydroxyalkanoate was poly-β-hydroxybutyrate. According to gene expression profile of NGB15, it was determined that Pseudomonas neustonica NGB15 produces PHA using the de novo fatty acid synthesis metabolic pathway. In conclusion, poly-β-hydroxybutyrate production by Pseudomonas neustonica NGB15 using a low-cost fermentation medium has been shown to be biotechnologically promising.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Petroleum-based plastics (synthetic plastic), which have wide applications in the household, pharmaceutical, and commercial sectors, have become an important raw material in modern society (Nanda et al. 2022). In 2019, global plastics production was 368 metric tonnes (Mt) and is expected to exceed 600 Mt in 2025 (Tyagi et al. 2021). On the other hand, it is observed that 10 million tons of synthetic plastic leaks into the oceans every year, and this has a harmful effect on the ocean ecosystem (Boucher and Billard 2019). Due to the developing technology and population growth, the demand for plastic and plastic derivative products is constantly increasing (Geyer et al. 2017). This causes serious environmental problems. It is known that petroleum-based plastics cause very serious ecological problems due to their low decomposition rate, the leakage of carcinogenic substances into the environment when exposed to heat, and the formation of toxic by-products in this process (Gironi and Piemonte 2011). There are several solutions to the environmental pollution caused by plastic waste. These are as follows: incineration, recycling practices, or producing and using biodegradable plastics (bioplastics) (Evode et al. 2021). The destruction of petroleum-based plastics by burning and the emergence of harmful gases such as hydrochloric acid and hydrogen cyanide during the process are methods that put the environment and human health at risk (Roy et al. 2021). Although recycling applications are seen as an effective solution, the process is tremendously slow, and the necessity of separating and then recycling plastics with different structures poses a problem for this application (Vogt et al. 2021). In addition to all these, the presence of additives such as fillers and colorants used in plastics limits the recycling of synthetic plastics. In order to reduce the harmful effects of petroleum-based plastics caused by overuse and accumulation, there is a need for environmentally friendly new polymers that are easily degradable and do not harm the environment when decomposed as an alternative to petroleum-based plastics (Naser et al. 2021). Polyhydroxyalkanoates (PHA), known as bioplastics, are seen as an alternative to synthetic plastics due to their physicochemical and mechanical properties (Alcantara et al. 2020). Unlike petroleum-based plastics, bioplastics are becoming popular recently due to their biodegradability, biocompatibility, environmentally friendly production processes, and wide range of applications (Albuquerque and Malafaia 2018).
Polyhydroxyalkonates are produced and stored by bacteria in the absence of essential nutrients such as phosphorus, nitrogen, and sulfur and when there is an excess of carbon source (Kumar et al. 2020). The interest in bioplastics is increasing day by day with the positive results obtained regarding the synthesis and industrial applications of biodegradable environmentally friendly plastics such as PHA by bacteria (Behera et al. 2022). Although bioplastics are environmentally friendly, easily biodegradable, and not harmful to human health, but the production of bioplastics process is more expensive than synthetic plastics (Kumar et al. 2020). The species Cupriavidus necator is widely studied for the biosynthesis and production of PHAs (Bellini et al. 2022). Recently, with the emergence of new processes and technologies for the production of economical PHAs, PHA production has been successfully performed in species such as Bacillus sp. (Yasin and Al-Mayaly 2021), Pseudomonas sp. (Kanavaki et al. 2021), Aeromonas hydrophila (Szacherska et al. 2022), Rhodopseudomonas palustris (Brown et al. 2022), Burkholderia sacchari (Oliveira-Filho et al. 2022), and Halomonas boliviensis (Arcila-Echavarría et al. 2022).
Although bioplastics are seen as an alternative to synthetic plastics recently, they need to be produced at a more cost-effective which is one of the major stumbling blocks in the expansion and growth of the PHA market (Koller et al. 2017). Several critical factors are considered important to make bacterial PHA production cost-effective. These:
-
(1)
Screening and selection of bacterial strains that have the potential to produce high amounts of PHA and even the discovery of new bacterial strains are required (Kumar et al. 2016)
-
(2)
Use of cheap carbon sources in the production process (Zahari et al. 2014)
-
(3)
Illuminating PHA synthesis pathways (Możejko-Ciesielska and Mostek 2019a)
The aim of this study is to determine and improve the optimum growing conditions for PHA production by a local strain for possible industrial usage. As a result, PHA production by P. neustonica strain NGB15 was evaluated, and its yield was enhanced by the optimization of some culture conditions. Subsequently, in an effort to increase PHA production while lowering fermentation costs, agro-residues including banana and peanut peels were assessed as alternative carbon sources in P. neustonica NGB15 fermentation for the first time.
Materials and methods
Collection of samples
The most important condition for PHA production in bacteria is the presence of a high carbon source in the culture medium. For this purpose, waste samples from the Erzurum sugar factory, which have a high carbon source content, were brought to the laboratory environment in sterile glass bottles under aseptic conditions for the isolation of PHA-producing bacteria.
Preparation of substrates
Banana and peanut peels were washed properly, followed by oven drying at 80 °C. The dried banana and peanut peels were ground into fine particles by blender and termed banana peel powder (BPP) and peanut peel powder (PPP) respectively.
Isolation and screening of PHA-producing bacteria
Sugar factory waste samples were added to the flask containing 100 ml of Tryptic Soy Broth (TSB) and incubated at 25 °C for 48 h. Then, serial dilutions were prepared, and the bacteria were purified (Baltaci et al. 2024). Since PHA production of bacteria absolutely depends on the content of the medium (high carbon source, low sulfur rate, etc.), pure cultured organisms were inoculated into Minimal Davis Broth (MDB) medium, which was used in previous literature data, to stimulate PHA production. Next, the PHA production potential of the bacteria was determined according to the protocol of Taran et al. (2011). The best PHA producer isolate was selected.
Determination of PHA production
PHA production was determined, using the procedure reported by Taran (2011). Briefly, 5 ml of culture was centrifuged, and the pellets were dissolved in the dH2O and incubated at 25 °C for an hour to cells’ total lysis. Then the lysate was centrifuged, and the same volume of ethanol and acetone was added to the pellet. The residue was dissolved in chloroform.
The mixture was kept at room temperature to evaporate the chloroform. After being treated with 5 mL of 17.8 M H2SO4, the resulting pellet was incubated in boiling water for 40 min. Following this stage, an ultraviolet–visible (UV–Vis) spectrophotometer (Shimadzu, UV 1800 240 V, Japan) was used to measure the generation of crotonic acid at OD235, with H2SO4 serving as a blank. Using polyhydroxybutyrate as a reference, the concentration of PHB in the sample was determined (Sigma-Aldrich).
Molecular characterization of best PHA producer isolate
The Promega WizardR Genomic DNA Purification Kit (A2360) was used for the genomic DNA isolation of the best strain. The 16S rRNA gene region was amplified using 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) primers (Baltaci 2022; Baltaci et al. 2020b). Using the pGEM-T Easy Cloning Vector (Promega, Southampton, UK), the PCR product was cloned into the Escherichia coli JM101 strain in accordance with the manufacturer’s instructions. The Macrogen Company (Amsterdam, Netherlands) performed the sequence analysis following the cloning (Adiguzel et al. 2019; Akbulut et al. 2022). After the 16S rRNA was acquired, its similarity rate was calculated, and GenBank accession numbers were obtained by comparing it to the other bacterial series found in GenBank and EzTaxon (http://blast.ncbi.nlm.nih and http://www.eztaxon.org). A phylogenetic tree was constructed with Mega4 software based on the 16S rDNA sequences (Baltaci and Adiguzel 2016; Baltaci et al. 2017).
Optimization of culture conditions
After it was detected the best PHA producer isolate, the culture conditions were optimized. For this, initial optimization experiments were carried out to determine the best waste carbon source and concentration and temperature. Then, pH and incubation time parameters were optimized (Baltaci et al. 2022). Detailed experimental conditions for the optimization studies are given Table 1.
PHA extraction
NGB15 was grown under optimum culture conditions. Then, the culture was centrifuged, the cells were washed with distilled water, and the cells were lyophilized. Lyophilized cell powder was suspended in 20 mL of chloroform and 20 mL of sodium hypochlorite solution (30%, pH 12.15). The suspended culture was incubated at 30 °C for 150 min and centrifuged at 30 °C for 20 min. After centrifugation, three different phases were formed. The bottom chloroform phase was carefully collected and recovered by PHA (80% methanol) precipitation and filtration (Saratale and Oh 2015).
ATR-FTIR analysis
The functional groups found in the bioplastics were identified by infrared (IR) spectra. FTIR analysis of the PHA sample was performed at the Eastern Anatolia High Technology Application and Research Center (DAYTAM). The purified PHA sample (2.5 mg) was analyzed by attenuated total reflectance (ATR) technique using a Nicolas iS50 spectrometer (Thermo Fisher Scientific, Waltham, USA). The absorption was scanned at a spectral range of 400–4000 cm−1 (Kurt-Kizildogan et al. 2021).
Determination of metabolic pathway of PHA production
To determine the metabolic pathways through which NGB15 produces PHA under the optimized conditions, the expression of genes involved in these metabolic pathways was determined by real-time PCR. For this purpose, NGB15 was grown under optimized culture conditions, and RNA isolation was performed using the RNeasy Mini Kit (Qiagene-Germany) in accordance with the manufacturer’s instructions. Then, cDNA was synthesized using ThermoScript™ RT-PCR System for First-Strand cDNA Synthesis Kit (Invitrogen-USA). Real-time PCR was performed with Maxima SYBR Green/ROX qPCR Master Mix (2 ×) (Qiagen, Germany) in Rotor-Gene Q 6000 Real-time PCR system (Qiagen, Hilden, Germany). The reaction was carried out in a 20-µL mixture for each tube containing 2 µL cDNA template, 10 µL master mix, 1 µL of each primer, and 6 µL sterilized nuclease-free water. The program was as follows: initial denaturation step at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing temperature for each primer for 30 s, and extension at 72 °C for 20 s. Fluorescence signals were measured after the annealing/extension step of each cycle. The primer list of target genes is given in Table 2. 16S rRNA gene was used for normalization and relative quantification.
Result and discussion
Isolation and screening PHA producer bacteria
A total of 15 bacteria with PHA-producing potential were isolated from waste sugar factory samples, and PHA production amounts were determined spectrophotometrically according to the Taran method (Table 3) (Taran 2011).
It was determined that the NGB15 had the highest PHA production potential; therefore, the next experiments were continued with this isolate. Then, molecular characterization of NGB15 was performed by 16S rRNA gene sequence analysis. As a result of 16S rRNA gene sequence analysis, it was determined that NGB15 belonged to P. neustonica with a 99.72% similarity rate. Neighbor-joining phylogenetic tree on the basis of 16S rRNA gene sequence data of NGB15 was constructed using the MEGA4 program. Pallidibacillus pasinlerensis was used as an out-group (Baltaci et al. 2020a). Bootstrap values based on 1000 replications are listed as percentages at branching points. Only bootstrap values > 50% are shown at nodes. The scale bar represented a 2% divergence (Fig. 1). In the literature, although there are many Pseudomonas species producing bioplastic (Manso et al. 2012; Zong et al. 2022), this study is the first study on bioplastic production from P. neustonica.
Optimization of culture conditions
Numerous studies have demonstrated that a variety of physicochemical parameters, including type and concentration of nitrogen and carbon sources, cultivation time, pH, temperature, and agitation affect the production of PHA (Anjum et al. 2016; Moreno et al. 2015; Tarrahi et al. 2020). Thus, the one-variable-at-a-time (OVAT) strategy was used to optimize some culture parameters, including substrate concentration, pH, temperature, and incubation time. Initially, different concentrations (from 10 to 40 g/L) of waste carbon sources (waste baklava sherbet, banana peel powder, and peanut shell powder) were tested. The highest PHA production (OD235 1.55) was observed in banana peel powder at 30 g/L concentration, and the lowest PHA production was observed in waste baklava sherbet (Fig. 2a). This decrease in PHA production may be due to the high sugar content in baklava sherbet inhibiting bacterial growth. C to N ratio is a very important factor affecting the efficiency of PHA production. Many studies reported that a high C to N ratio positively affects PHA production (Dash et al. 2020; Zhao et al. 2021). While the C to N ratio of peanut shells is higher than the banana peels, higher PHA production was achieved with banana peel powder. This situation can be explained as the high starch content of banana peels enhanced the production of PHA. Temperature plays a crucial role in PHA production by changing the physiology and diversity of microbial flora. When the results were analyzed, it was observed that the NGB15 produced PHA in a wide temperature range (15–35 °C) and the best PHA production (OD235 1.73) was found to be at 25 °C (Fig. 2b). PHA production efficiency was found to be low at the high temperature (> 40 °C). This is probably due to the decreased activity of enzymes involved in PHA biosynthesis at these temperatures. Because high temperature reduces the metabolic activity (enzyme activity) of microorganisms, it could create a change in PHA content or reduce PHA production efficiency (Mahato et al. 2021).
pH is also known to affect PHA production in microbes. As shown in Fig. 2c, the maximum PHA biosynthesis was at pH 8 (OD235 1.87). Alkaline pH (7–10) favored more PHA accumulation than acidic pH (4–7). Many studies reported that high pH was more suitable for PHA production. For example, Jau et al. reported that the best PHA production was achieved at pH 9 by Spirulina platensis (Jau et al. 2005). In another study, it was shown that biosynthesis of PHA production was observed at pH 8 (Ansari and Fatma 2016).
Also, PHA biosynthesis by strain NGB15 was tested from 1 to 7 days. Maximum PHA accumulation was observed on the fourth day (OD235 1.99) (Fig. 2d). A gradual decrease in PHA production was observed after the fourth day. This decrease is probably due to the use of the produced PHA as a carbon and energy source in the following incubation periods.
After the optimization studies, optimal culture conditions were determined as 30 g/L banana peel powder, 25 °C temperature, pH 8, and 4-day incubation time. Under optimized conditions, 3.34 g/L PHA was produced. It is known that under certain conditions, Pseudomonas can produce PHA and store it intracellularly in inclusion bodies. Therefore, there are many studies on PHA production with Pseudomonas and other strains (Table 4).
Mahato et al. achieved 5.9 g/L production with P. aeruginosa from groundnut oil, and in another study conducted by Qin et al., 3.98 g/L PHA production was performed with P. putida from glucose. These amounts are higher than our production. However, in this study, a more cost-effective production was achieved by using waste materials instead of glucose. Since production cost is the most important disadvantage of bioplastics, cost-effective production is an important point.
Characterization of PHA
FTIR analysis
PHAs often have a similar formula with different R-hydroxyalkanoic acid groups attached and classified into three groups: short chain length (scl), medium chain length (mcl), and long chain length (lcl) depending on the structure, number of carbon atoms, and branching of the chain. FTIR analyses were performed to characterize the produced PHA and determine which chemical groups it contains (Fig. 3). As seen in Fig. 3, Major peaks were observed at wave numbers 1723, 1277, 1261,1097, 1054, and 993. According to these results, it was detected that the PHA produced by the NGB15 was short chain length (scl). In the literature, it was reported that a peak at 1720–1740 cm−1 represents the presence of an ester group (Bhagowati et al. 2015; Özgören et al. 2018), The peak at approximately 1280 cm−1 shows C–O–C stretching, and approximately 1263 cm−1 corresponds both C–O–C stretching and CH deformation. Also, 1228 cm−1 indicates the helical structure of the polymer. The peaks at almost 1185 cm−1 and 1130 cm−1 are determinative of asymmetric and symmetric vibrations of the C–O–C group. In addition, bands at approximately 1100 cm−1 and 1042 cm−1 show C–O–C stretching and C-CH3 stretching, respectively (Özgören et al. 2018). When these results were compared with the literature, it was determined that the type of PHA produced by NGB15 was poly-β-hydroxybutyrate (PHB) (Table 5).
Detection of the metabolic pathway of NGB15
It has been reported that microbial PHA synthesis has three different metabolic pathways (Khatami et al. 2021) (Figure 4).
To determine which metabolic pathway NGB15 used to produce PHA, the expression of genes playing a role in these pathways was analyzed. The highest expression was observed in fabD, phaG, and phaC genes. According to these results, it was determined that P. neustonica NGB15 produces PHA using the de novo fatty acid synthesis metabolic pathway (Pathway III) (Fig. 5). In a study with P. putida KT2440, it was reported that PHA production was performed using the de novo fatty acid synthesis metabolic pathway (Możejko-Ciesielska and Mostek 2019b).
Conclusion
Environmental pollution caused by conventional petroleum-based plastics has reached its peak. Therefore, with the recent projected reductions in fossil fuel resources, stringent regulations, and public awareness, the search for biodegradable and sustainable plastics is encouraging. Microbial polyhydroxyalkanoates (PHAs) are prime candidates for “green” alternatives to petroleum-based plastics. However, PHAs need to be produced at more affordable costs. The use of agricultural waste as a carbon source contributes to the preparation of a low-cost medium for PHA production. In this study, PHA production was achieved for the first time with the P. neustonica NGB15 using agricultural waste. Then produced PHA was characterized as poly-β-hydroxybutyrate (PHB). PHB production by P. neustonica NGB15 using a low-cost fermentation medium has been shown to be biotechnologically promising.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abid S, Raza ZA, Hussain T (2016) Production kinetics of polyhydroxyalkanoates by using gamma ray mutant strain EBN-8 cultured on soybean oil. 3 Biotech 6:142. https://doi.org/10.1007/s13205-016-0452-4
Adiguzel G, Faiz O, Sisecioglu M et al (2019) A novel endo-β-1,4-xylanase from GC25; purification, characterization and application in clarification of fruit juices. Int J Biol Macromol 129:571–578. https://doi.org/10.1016/j.ijbiomac.2019.02.054
Akbulut S, Baltaci MO, Adiguzel G, Adiguzel A (2022) Identification and potential biotechnological characterization of lactic acid bacteria isolated from white cheese samples. J Pure Appl Microbio 16(4). https://doi.org/10.22207/Jpam.16.4.66
Albuquerque PBS, Malafaia CB (2018) Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int J Biol Macromol 107:615–625. https://doi.org/10.1016/j.ijbiomac.2017.09.026
Alcantara JMG, Distante F, Storti G, Moscatelli D, Morbidelli M, Sponchioni M (2020) Current trends in the production of biodegradable bioplastics: the case of polyhydroxyalkanoates. Biotechnol Adv 42:107582. https://doi.org/10.1016/j.biotechadv.2020.107582
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): screening, optimization and characterization. PLoS ONE 11(6):e0158168
Arcila-Echavarría DC, Lu-Chau TA, Gómez-Vanegas NA et al (2022) Optimization of nutritional and operational conditions for producing PHA by the halophilic bacterium halomonas boliviensis from oil palm empty fruit bunch and gluten hydrolysates. Waste Biomass Valor 13:1589–1597. https://doi.org/10.1007/s12649-021-01605-9
Baltaci MO (2022) Enhancement of cellulase production by co-culture of Streptomyces ambofaciens OZ2 and Cytobacillus oceanisediminis OZ5 isolated from rumen samples. Biocatal Biotransfor 40(2):144–152. https://doi.org/10.1080/10242422.2022.2038581
Baltaci MO, Adiguzel A (2016) Isolation, identification and molecular characterization of cellulolytic bacteria from rumen samples collected from erzurum slaughter house. Turkey Res J Biotechnol 11(2):32–38
Baltaci MO, Genc B, Arslan S, Adiguzel G, Adiguzel A (2017) Isolation and characterization of thermophilic bacteria from geothermal areas in Turkey and preliminary research on biotechnologically important enzyme production. Geomicrobiol J 34(1):53–62. https://doi.org/10.1080/01490451.2015.1137662
Baltaci MO, Ay H, Akbulut S et al (2020a) Bacillus pasinlerensis sp. nov., a thermophilic bacterium isolated from a hot spring in Turkey. Int J Syst Evol Micr 70(6):3865–3871
Baltaci MO, Orak T, Taskin M, Adiguzel A, Ozkan H (2020b) Enhancement of amylase and lipase production from 016 using waste chicken feathers as peptone source. Waste Biomass Valori 11(5):1809–1819. https://doi.org/10.1007/s12649-018-0468-6
Baltaci MO, Omeroglu MA, Albayrak S, Adiguzel G, Adiguzel A (2022) Production of endoglucanase by OB24 using waste melon peels as substrate. An Acad Bras Cienc 94 ARTN e20220151. https://doi.org/10.1590/0001-3765202220220151
Baltaci MO, Omeroglu MA, Ozkan H, Taskin M, Adiguzel A (2024) Enhanced biodegradation of crude oil contamination by indigenous bacterial consortium under real conditions. Biocatal Biotransfor 42(1):56–67. https://doi.org/10.1080/102424222231592
Behera S, Priyadarshanee M, Vandana, Das S (2022) Polyhydroxyalkanoates, the bioplastics of microbial origin: properties, biochemical synthesis, and their applications. Chemosphere 294 ARTN 133723. https://doi.org/10.1016/j.chemosphere.2022.133723
Bellini S, Tommasi T, Fino D (2022) Poly (3-hydroxybutyrate) biosynthesis by Cupriavidus necator: a review on waste substrates utilization for a circular economy approach. Bioresource Technology Reports 17:100985
Bhagowati P, Pradhan S, Dash HR, Das S (2015) Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by spp. Biosci Biotech Bioch 79(9):1454–1463. https://doi.org/10.1080/09168451.2015.1034651
Bhatia SK, Gurav R, Choi TR et al (2019) Bioconversion of plant biomass hydrolysate into bioplastic () using 5119. Bioresource Technol 271:306–315. https://doi.org/10.1016/j.biortech.2018.09.122
Boucher J, Billard G (2019) The challenges of measuring plastic pollution. Field actions science reports. J Field Actions (special issue 19):68–75
Brown B, Immethun C, Alsiyabi A, Long D, Wilkins M, Saha R (2022) Heterologous phasin expression in Rhodopseudomonas palustris CGA009 for bioplastic production from lignocellulosic biomass. Metabolic Eng Commun 14:e00191
Chaudhry WN, Jamil N, Ali I, Ayaz MH, Hasnain S (2011) Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann Microbiol 61(3):623–629. https://doi.org/10.1007/s13213-010-0181-6
Dash A, Mohanty S, Samantaray D (2020) Effect of carbon/nitrogen ratio on polyhydroxyalkanoates production by Bacillus species under submerged fermentation. J Environ Biol 41(1):118–124
de Meneses L, Pereira JR, Sevrin C et al (2020) Pseudomonas chlororaphis as a multiproduct platform: conversion of glycerol into high-value biopolymers and phenazines. New Biotechnol 55:84–90. https://doi.org/10.1016/j.nbt.2019.10.002
Evode N, Qamar SA, Bilal M, Barceló D, Iqbal HM (2021) Plastic waste and its management strategies for environmental sustainability. Case Stud Chem Environ Eng 4:100142
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9:1–9
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3(7) ARTN e1700782. https://doi.org/10.1126/sciadv.1700782
Gironi F, Piemonte V (2011) Bioplastics and petroleum-based plastics: strengths and weaknesses. Energ Source Part A 33(21):1949–1959. https://doi.org/10.1080/15567030903436830
Hiroe A, Tsuge K, Nomura CT, Itaya M, Tsuge T (2012) Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly [(R)-3-hydroxybutyrate] in genetically engineered Escherichia coli. Appl Environ Microb 78(9):3177–3184
Hiroe A, Ushimaru K, Tsuge T (2013) Characterization of polyhydroxyalkanoate (PHA) synthase derived from Delftia acidovorans DS-17 and the influence of PHA production in Escherichia coli. J Biosci Bioeng 115(6):633–638
Jau M-H, Yew S-P, Toh PS et al (2005) Biosynthesis and mobilization of poly (3-hydroxybutyrate)[P (3HB)] by Spirulina platensis. Int J Biol Macromol 36(3):144–151
Kanavaki I, Drakonaki A, Geladas ED, Spyros A, Xie H, Tsiotis G (2021) Polyhydroxyalkanoate (PHA) production in sp. phDV1 strain grown on phenol as carbon sources. Microorganisms 9(8) ARTN 1636. https://doi.org/10.3390/microorganisms9081636
Khamkong T, Penkhrue W, Lumyong S (2022) Optimization of production of polyhydroxyalkanoates (PHAs) from newly isolated sp. strain HD34 by response surface methodology. Processes 10(8) ARTN 1632. https://doi.org/10.3390/pr10081632
Khatami K, Perez-Zabaleta M, Owusu-Agyeman I, Cetecioglu Z (2021) Waste to bioplastics: how close are we to sustainable polyhydroxyalkanoates production? Waste Manag 119:374–388
Koller M, Maršálek L, de Sousa Dias MM, Braunegg G (2017) Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol 37:24–38
Kumar M, Gupta A, Thakur IS (2016) Carbon dioxide sequestration by chemolithotrophic oleaginous bacteria for production and optimization of polyhydroxyalkanoate. Bioresource Technol 213:249–256
Kumar M, Rathour R, Singh R, et al. (2020) Bacterial polyhydroxyalkanoates: opportunities, challenges, and prospects. J Clean Prod 263 ARTN 121500. https://doi.org/10.1016/j.jclepro.2020.121500
Kurt-Kizildogan A, Türe E, Okay S et al (2021) Improved production of poly(3-hydroxybutyrate) by extremely halophilic archaeon sp. TG1 by utilization of rCKT3eng-treated sugar beet pulp. Biomass Conv Bioref 13:10911–10921. https://doi.org/10.1007/s13399-021-02011-w
Mahato RP, Kumar S, Singh P (2021) Optimization of growth conditions to produce sustainable polyhydroxyalkanoate bioplastic by EO1. Front Microbiol 12 ARTN 711588. https://doi.org/10.3389/fmicb.2021.711588
Manso IM, Ibáñez I, Sáez LP et al (2012) Assimilation of cyanide and synthesis of bioplastics by CECT5344. Febs J 279:391–391
Moreno P, Yañez C, Cardozo NSM, Escalante H, Combariza MY, Guzman C (2015) Influence of nutritional and physicochemical variables on PHB production from raw glycerol obtained from a Colombian biodiesel plant by a wild-type Bacillus megaterium strain. New Biotechnol 32(6):682–689
Możejko-Ciesielska J, Mostek A (2019a) A 2D-DIGE-based proteomic analysis brings new insights into cellular responses of Pseudomonas putida KT2440 during polyhydroxyalkanoates synthesis. Microb Cell Fact 18(1):1–13
Możejko-Ciesielska J, Mostek A (2019b) Time-course proteomic analysis of Pseudomonas putida KT2440 during mcl-polyhydroxyalkanoate synthesis under nitrogen deficiency. Polymers-Basel 11(5):748
Nanda S, Patra BR, Patel R, Bakos J, Dalai AK (2022) Innovations in applications and prospects of bioplastics and biopolymers: a review. Environ Chem Lett 20(1):379–395. https://doi.org/10.1007/s10311-021-01334-4
Naser AZ, Deiab DBM (2021) Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review. Rsc Adv 11(28):17151–17196. https://doi.org/10.1039/d1ra02390j
Oliveira-Filho ER, de Macedo MA, Lemos AC et al (2022) Engineering Burkholderia sacchari to enhance poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)[P (3HB-co-3HHx)] production from xylose and hexanoate. Int J Biol Macromol 213:902–914
Özgören T, Pinar O, Bozdag G et al (2018) Assessment of poly(3-hydroxybutyrate) synthesis from a novel obligate alkaliphilic and generation of its composite scaffold via electrospinning. Int J Biol Macromol 119:982–991. https://doi.org/10.1016/j.ijbiomac.2018.08.014
Qin RL, Zhu YZ, Ai MM, Jia XQ (2022) Reconstruction and optimization of a microbial consortium for mcl-PHA production from lignocellulosic biomass. Front Bioeng Biotech 10 ARTN 1023325. https://doi.org/10.3389/fbioe.2022.1023325
Rebocho AT, Pereira JR, Neves LA et al (2020) Preparation and characterization of films based on a natural p (3hb)/mcl-pha blend obtained through the co-culture of cupriavidus necator and pseudomonas citronellolis in apple pulp waste. Bioengineering 7(2):34
Reddy MV, Watanabe A, Onodera R et al (2020) Polyhydroxyalkanoates (PHA) production using single or mixture of fatty acids with Bacillus sp. CYR1: identification of PHA synthesis genes. Bioresource Technology Reports 11:100483
Roy PS, Garnier G, Allais F, Saito K (2021) Strategic approach towards plastic waste valorization: challenges and promising chemical upcycling possibilities. Chemsuschem 14(19):4007–4027. https://doi.org/10.1002/cssc.202100904
Saratale GD, Oh M-K (2015) Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int J Biol Macromol 80:627–635
Szacherska K, Moraczewski K, Czaplicki S, Oleskowicz-Popiel P, Mozejko-Ciesielska J (2022) Conversion of short and medium chain fatty acids into novel polyhydroxyalkanoates copolymers by sp. AC_01. Materials 15(13) ARTN 4482. https://doi.org/10.3390/ma15134482
Taran M (2011) Utilization of petrochemical wastewater for the production of poly(3-hydroxybutyrate) by sp IRU1. J Hazard Mater 188(1–3):26–28. https://doi.org/10.1016/j.jhazmat.2011.01.036
Tarrahi R, Fathi Z, Seydibeyoğlu MÖ, Doustkhah E, Khataee A (2020) Polyhydroxyalkanoates (PHA): from production to nanoarchitecture. Int J Biol Macromol 146:596–619
Tyagi B, Takkar S, Meena R, Thakur IS (2021) Production of polyhydroxybutyrate (PHB) by sp. ISTM3 isolated from Mawsmai cave utilizing molasses as carbon source. Environ Technol Inno 24 ARTN 101854. https://doi.org/10.1016/j.eti.2021.101854
Vogt BD, Stokes KK, Kumar SK (2021) Why is recycling of postconsumer plastics so challenging? Acs Appl Polym Mater 3(9):4325–4346. https://doi.org/10.1021/acsapm.1c00648
Wang X, Lin L, Dong J et al (2018) Simultaneous improvements of Pseudomonas cell growth and polyhydroxyalkanoate production from a lignin derivative for lignin-consolidated bioprocessing. Appl Environ Microb 84(18):e01469-e1518
Yasin AR, Al-Mayaly IK (2021) Study of the fermentation conditions of the strain ARY73 to produce polyhydroxyalkanoate (PHA) from glucose. J Ecol Eng 22(8):41–53. https://doi.org/10.12911/22998993/140326
Zahari MAKM, Abdullah SSS, Roslan AM, Ariffin H, Shirai Y, Hassan MA (2014) Efficient utilization of oil palm frond for bio-based products and biorefinery. J Clean Prod 65:252–260
Zhao L, Bao M, Zhao D, Li F (2021) Correlation between polyhydroxyalkanoates and extracellular polymeric substances in the activated sludge biosystems with different carbon to nitrogen ratio. Biochem Eng J 176:108204
Zong QJ, Xu T, Liu H, et al. (2022) Microbial valorization of lignin to bioplastic by genome-reduced. Front Microbiol 13 ARTN 923664. https://doi.org/10.3389/fmicb.2022.923664
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research was financially supported by the Scientific and Technological Research Council of Türkiye (TÜBİTAK, BİDEP 2218, Grant No: 118C534).
Author information
Authors and Affiliations
Contributions
Nurdan Gönül Baltacı: conceived and designed the analysis, performed the analysis, writing—original draft. Mustafa Özkan Baltaci: writing—original draft, performed the analysis. Arzu Görmez: methodology, conceptualization. Serkan Örtücü: methodology, conceptualization.
Corresponding author
Ethics declarations
Ethics approval
This article does not involve any study with human participants or animals to be performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
All authors consent when it is submitted.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baltacı, N.G., Baltacı, M.Ö., Görmez, A. et al. Green alternatives to petroleum-based plastics: production of bioplastic from Pseudomonas neustonica strain NGB15 using waste carbon source. Environ Sci Pollut Res 31, 31149–31158 (2024). https://doi.org/10.1007/s11356-024-33309-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33309-7