Abstract
Purpose
To detect the expression level and significance of SOX10 in human bladder cancer.
Methods
Immunohistochemical analyses were performed to assess SOX10 protein level using a bladder cancer tissue microarray (including 59 spots of cancer tissues and 46 spots of paired normal tissues) and 31 specimens and to define the relationship between SOX10 and clinicopathological bladder cancer characteristics in patients. SOX10 protein and mRNA levels in bladder cancer cell lines (T24, 5637, BIU87, EJ) and transitional cell papilloma cell line (RT4) were tested by western blotting and quantitative real-time PCR (q-PCR), respectively. Cell Counting Kit-8 (CCK-8) and colony formation assays were performed to investigate bladder cancer cell proliferation after SOX10 knockdown. The effect of SOX10 on cell migration and invasion was analyzed by Transwell and Matrigel assays. Kaplan–Meier survival curves and Cox regression analyses were used to evaluate SOX10 prognostic significance for bladder cancer patients. The mechanisms by which SOX10 promote bladder cancer progression were examined by western blotting.
Results
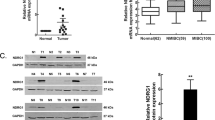
SOX10 protein was upregulated in 74.4% of bladder cancer tissues compared with adjacent normal tissues (32.6%). SOX10 protein was also upregulated in malignant cell lines. In addition, high SOX10 expression was related with clinical stage (P = 0.008), T stage (P = 0.004), histological grade (P = 0.002) and lymph node metastasis (P = 0.006). Kaplan–Meier survival curves and Cox regression analyses showed that SOX10 functioned as an independent prognostic factor for overall survival. SOX10 knockdown in bladder cancer cells significantly impacted proliferation, migration and invasion, and SOX10 might promote bladder cancer progression by altering β-catenin and Met expression.
Conclusion
SOX10 was over-expressed in bladder cancer and promoted malignant bladder cancer cell behaviors. SOX10 has potential as a molecular target for bladder cancer treatment.






Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Apolo AB, Vogelzang NJ, Theodorescu D. New and promising strategies in the management of bladder cancer. Am Soc Clin Oncol. 2015;35:105–12.
Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120(3):408–14.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38.
Hennings H, Glick AB, Greenhalgh DA, Morgan DL, Strickland JE, Tennenbaum T, et al. Critical aspects of initiation, promotion, and progression in multistage epidermal carcinogenesis. Proc Soc Exp Biol Med. 1993;202(1):1–8.
Ramos JR, Pabijan J, Garcia R, Lekka M. The softening of human bladder cancer cells happens at an early stage of the malignancy process. Beilstein J Nanotechnol. 2014;5:447–57.
Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346(6281):245–50.
Watanabe Y, Broders-Bondon F, Baral V, Paul-Gilloteaux P, Pingault V, et al. Sox10 and Itgb1 interaction in enteric neural crest cell migration. Dev Biol. 2013;379(1):92–106.
Miyahara K, Kato Y, Koga H, Dizon R, Lane GJ, Suzuki R, et al. Visualization of enteric neural crest cell migration in SOX10 transgenic mouse gut using time-lapse fluorescence imaging. J Pediatr Surg. 2011;46(12):2305–8.
Mollaaghababa R, Pavan WJ. The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene. 2003;22(20):3024–34.
Inoue K, Tanabe Y, Lupski JR. Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann Neurol. 1999;46(3):313–8.
Izumi Y, Musha I, Suzuki E, Iso M, Jinno T, Horikawa R, et al. Hypogonadotropic hypogonadism in a female patient previously diagnosed as having waardenburg syndrome due to a sox10 mutation. Endocrine. 2015;49(2):553–6.
Okamura K, Oiso N, Tamiya G, Makino S, Tsujioka D, Abe Y, et al. Waardenburg syndrome type IIE in a Japanese patient caused by a novel missense mutation in the SOX10 gene. J Dermatol. 2015;42(12):1211–2.
Wenzhi H, Ruijin W, Jieliang L, Xiaoyan M, Haibo L, Xiaoman W, et al. Heterozygous deletion at the SOX10 gene locus in two patients from a Chinese family with Waardenburg syndrome type II. Int J Pediatr Otorhinolaryngol. 2015;79(10):1718–21.
Panaccione A, Chang MT, Carbone BE, Guo Y, Moskaluk CA, Virk RK, et al. NOTCH1 and SOX10 are essential for proliferation and radiation resistance of cancer stem-like cells in adenoid cystic carcinoma. Clin Cancer Res. 2016;22(8):2083–95.
Kwon AY, Heo I, Lee HJ, Kim G, Kang H, Heo JH, et al. Sox10 expression in ovarian epithelial tumors is associated with poor overall survival. Virchows Arch. 2016;468(5):597–605.
Schmitt AC, Cohen C, Siddiqui MT. Expression of SOX10 in salivary gland oncocytic neoplasms: a review and a comparative analysis with other immunohistochemical markers. Acta Cytol. 2015;59(5):384–90.
Lopez-Anido C, Sun G, Koenning M, Srinivasan R, Hung HA, Emery B, et al. Differential Sox10 genomic occupancy in myelinating glia. Glia., 2015;63(11):1897–914.
Zhou D, Bai F, Zhang X, Hu M, Zhao G, Zhao Z, et al. SOX10 is a novel oncogene in hepatocellular carcinoma through Wnt/beta-catenin/TCF4 cascade. Tumour Biol. 2014;35(10):9935–40.
Tong X, Li L, Li X, Heng L, Xhong L, Su X, et al. SOX10, a novel HMG-box-containing tumor suppressor, inhibits growth and metastasis of digestive cancers by suppressing the Wnt/beta-catenin pathway. Oncotarget. 2014;5(21):10571–83.
Han B, Luan L, Xu Z, Wu B. Clinical significance and biological roles of CRKL in human bladder carcinoma. Tumour Biol. 2014;35(5):4101–6.
Sutherland JM, Sobinoff AP, Fraser BA, Redgrove KA, Davidson TL, Siddall NA, et al. RNA binding protein Musashi-1 directly targets Msi2 and Erh during early testis germ cell development and interacts with IPO5 upon translocation to the nucleus. FASEB J. 2015;29(7):2759–68.
Bondurand N, Kobetz A, Pingault V, Lemort N, Encha-razavi F, Couly G, et al. Expression of the SOX10 gene during human development. FEBS Lett. 1998;432(3):168–72.
Lee KE, Nam S, Cho EA, Seong I, Lima JK, Lee S, et al. Identification of direct regulatory targets of the transcription factor Sox10 based on function and conservation. BMC Genom. 2008;9:408.
Bondurand N, Kuhlbrodt K, Pingault V, Enderich J, Sajus M, Tommerup N, et al. A molecular analysis of the yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum Mol Genet. 1999;8(9):1785–9.
Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000;107(1):1–6.
Sham MH, Lui VC, Fu M, Chen B, Tam PK. SOX10 is abnormally expressed in aganglionic bowel of Hirschsprung’s disease infants. Gut. 2001;49(2):220–6.
Bondurand N, Girard M, Pingault V, Lemort N, Dubourg O, Goossens M. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10. Hum Mol Genet. 2001;10(24):2783–95.
Pingault V, Girard M, Bondurand N, Dorkins H, Van Maldergem L, Mowat D, et al. SOX10 mutations in chronic intestinal pseudo-obstruction suggest a complex physiopathological mechanism. Hum Genet. 2002;111(2):198–206.
Paratore C, Eichenberger C, Suter U, Sommer L. Sox10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum Mol Genet. 2002;11(24):3075–85.
McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233(2):430–44.
Bannykh SI, Stolt CC, Kim J, Perry A, Wegner M. Oligodendroglial-specific transcriptional factor SOX10 is ubiquitously expressed in human gliomas. J Neurooncol. 2006;76(2):115–27.
Addo-Yobo SO, Straessle J, Anwar A, Donson AM, Kleinschmidt-demasters BK, Foreman NK. Paired overexpression of ErbB3 and Sox10 in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2006;65(8):769–75.
Yokoyama S, Takeda K, Shibahara S. Functional difference of the SOX10 mutant proteins responsible for the phenotypic variability in auditory-pigmentary disorders. J Biochem. 2006;140(4):491–9.
Ferletta M, Uhrbom L, Olofsson T, Ponten F, Westermark B. Sox10 has a broad expression pattern in gliomas and enhances platelet-derived growth factor-B–induced gliomagenesis. Mol Cancer Res. 2007;5(9):891–7.
Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32(9):1291–8.
Flammiger A, Besch R, Cook AL, Maier T, Sturm RA, Berking C. SOX9 and SOX10 but not BRN2 are required for nestin expression in human melanoma cells. J Invest Dermatol. 2009;129(4):945–53.
Blochin E, Nonaka D. Diagnostic value of Sox10 immunohistochemical staining for the detection of metastatic melanoma in sentinel lymph nodes. Histopathology. 2009;55(5):626–8.
Zhao Y, Liu ZG, Tang J, Zou RF, Chen XY, Jiang GM, et al. High expression of Sox10 correlates with tumor aggressiveness and poor prognosis in human nasopharyngeal carcinoma. Onco Targets Ther. 2016;9:1671–7.
Mohamed A, Gonzalez RS, Lawson D, Wang J, Cohen C. SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl Immunohistochem Mol Morphol. 2013;21(6):506–10.
Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26(8):1041–50.
Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012;14(8):882–90.
Ponder BA. Cancer genetics. Nature. 2001;411(6835):336–41.
Bartek J. DNA damage response, genetic instability and cancer: from mechanistic insights to personalized treatment. Mol Oncol. 2011;5(4):303–7.
Groner AC, Cato L, de Tribolet-Hardy J, Bernasocchi T, Janouskova H, Melchers D, et al. TRIM24 Is an Oncogenic Transcriptional Activator in Prostate Cancer. Cancer Cell. 2016;29(6):846–58.
Acknowledgements
We thank the Department of Pathology of Peking University People’s Hospital for their technology support of immunohistochemistry and staining evaluation.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81472393) and Beijing Natural Science Foundation (No. 7152149).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Additional information
H. Yin, and C. Qin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yin, H., Qin, C., Zhao, Y. et al. SOX10 is over-expressed in bladder cancer and contributes to the malignant bladder cancer cell behaviors. Clin Transl Oncol 19, 1035–1044 (2017). https://doi.org/10.1007/s12094-017-1641-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1641-2