Abstract
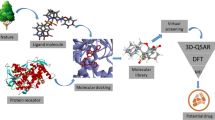
The diseases caused by dermatophytes are common among several other infections which cause serious threat to human health. It is evident that enzyme squalene epoxidase is responsible for prolonged dermatophyte infection and it is appealing to note that this enzyme is also responsible for fatty acid synthesis in these groups of fungi. In the present study, terbinafine drug which targets enzyme squalene epoxidase has been explored to design its various novel analogues. The present study suggests that many more prominent drug analogues could be constituted which may be crucial towards designing new drug candidates. In the present study, we have designed a series of such analogues viz. [(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)(naphthalen-1-ylmethyl)amine, N-[8-({[(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)amino}methyl)naphthalen-1-yl]-2-(sulfoamino) acetamide, {[4-(dihydroxyamino)-8-({[(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)amino}methyl)naphthalen-1-yl]sulfanyl}methanol and (R)-{[4-({[(2E,6R)-6,7-dimethyloct-2-en-4-yn-1-yl](methyl)amino}methyl)-5-[(hydroxysulfamoyl)amino]naphthalen-1-yl]amino}sulfinic acid. Moreover, further by molecular docking approach the binding between enzyme and designed analogues was further analysed. The present preliminary report suggested a considerably good docking interaction score of −338.75 kcal/mol between terbinafine and squalene epoxidase from Trichophyton rubrum. This preliminary study implies that few designed candidate ligands can be effectual towards the activity of this enzyme and can play crucial role in pathogenesis control of T. rubrum.








Similar content being viewed by others
References
Wang W, Chunquan S, Xiaoying C, Haitao J, Zhenyuan M, Jianzhong Y, Wannian Z (2009) Design, synthesis, and antifungal activity of novel conformationally restricted triazole derivatives. Arch Pharm Chem Life Sci 342:732–739. doi:10.1002/ardp.200900103
Cheung RC, Wong JH, Pan WL, Chan YS, Yin CM, Dan XL, Wang HX, Fang EF, Lam SK, Ngai PH, Xia LX, Liu F, Ye XY, Zhang GQ, Liu QH, Sha O, Lin P, Ki C, Bekhit AA, Ael-D Bekhit, Wan DC, Ye XJ, Xia J, Ng TB (2014) Antifungal and antiviral products of marine organisms. Appl Microbiol Biotechnol 98:3475–3494. doi:10.1007/s00253-014-5575-0
Regina L, Sandra F, Vlasta K, Natascha S, Eva P, Kathrin W, Mojca L, Karina L, Christoph R, Ivan H, Friederike T (2003) Molecular mechanism of terbinafine resistance in Saccharomyces cerevisiae. Antimicrob Agents Chemother 47:3890–3900. doi:10.1128/AAC.47.12.3890-3900.2003
Favre B, Ryder NS (1996) Characterization of squalene epoxidase activity from the dermatophyte Trichophyton rubrum and its inhibition by terbinafine and other antimycotic agents. Antimicrob Agents Chemother 40:443–447
Marcin N, Marcin H, Lucjan SW, Piotr S, Dariusz MP, Michal L, Krzysztof G, Leszek R (2011) Detailed mechanism of squalene epoxidase inhibition by terbinafine. J Chem Inf Model 51:455–462. doi:10.1021/ci100403b
Petranyi G, Meingassner JG, Mieth H (1987) Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother 31:1365–1368. doi:10.1128/AAC.31.9.1365
de Ludmila MB, Patrícia CS, Talles PP, Milene AR, Patrícia SC, Danielle GS, Daniel AS (2013) IFN-γ impairs Trichophyton rubrum proliferation in a murine model of dermatophytosis through the production of IL-1β and reactive oxygen species. Med Mycol 52:293–302. doi:10.1093/mmy/myt011
Tuukka S, Teijo IS, Nora MH, Janne TB, Pertti JN, Mika S, Klaus TO, Kari L (2015) Effects of terbinafine and itraconazole on the pharmacokinetics of orally administered tramadol. Eur J Clin Pharmacol. doi:10.1007/s00228-014-1799-2
Bhakti P, Meena AK, Pissurlenkar R, Mamata J, Evans CC, Sudha S (2010) Probing molecular level interaction of antifungal drugs with model membranes by molecular modeling, multinuclear NMR and DSC methods. Int J Cur Pharm Res 2:47–56
Prashant SK, Meenakshi ND, Vithal MK (2008) Design, synthesis, antifungal activity, and ADME prediction of functional analogues of terbinafine. Med Chem Res 18:421–432. doi:10.1007/s00044-008-9138-8
Che X, Sheng C, Wang W, Cao Y, Xu Y, Ji H, Dong G, Miao Z, Yao J, Zhang W (2009) New azoles with potent antifungal activity: design, synthesis and molecular docking. Eur J Med Chem 44:4218–4226. doi:10.1016/j.ejmech.2009.05.018
Francis G, Remi G, Cedric L, Fabrice P, Carine P, Marc LB, Patrice LP (2009) Synthesis and structure–activity relationships of 2-phenyl-1-[(pyridinyland piperidinylmethyl)amino]-3-(1H-1,2,4-triazol-1-yl)propan-2-ols as antifungal agents. Bioorg Med Chem Lett 19:301–304. doi:10.1016/j.bmcl.2008.11.101
Nalu TAP, Fernanda CAM, Antonio R, Nilce MMR (2010) Dermatophytes: host-pathogen interaction and antifungal resistance. An Bras Dermatol 85:657–667
Yan Z, Shichong Y, Renwu L, Qingjie Z, Xiang L, Maocheng W, Ting H, Xiaoxun C, Honggang Hu, Qiuye W (2014) Synthesis, antifungal activities and molecular docking studies of novel 2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propyl dithiocarbamates. Eur J Med Chem 74:366–374. doi:10.1016/j.ejmech.2014.01.009
Nalu TAP, Pablo RS, Juliana PF, Henrique CSS, Fernanda GP, Fernanda CAM, Diana EG, Fernando S, Rodrigo AC, Mendelson M, Jeny RCS, Roseli AF, Antonio R, Nilce MMR (2010) Transcriptional profiling reveals the expression of novel genes in response to various stimuli in the human dermatophyte Trichophyton rubrum. BMC Microbiol 10:39. doi:10.1186/1471-2180-10-39
Anke B, Ekaterina S, Gernot G, Christoph H, Susann S, Peter S, Andrew H, Marius F, Andreas P, Karol S, Marc F, Ivo P, Steffen P, Marco G, Robert W, Wenjun L, Olaf K, Volker S, Christian H, Bernhard H, Theodore CW, Matthias P, Reinhard G, Joseph H, Johannes W, Peter FZ, Michel M, Axel AB (2011) Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genom Biol 12:R7. doi:10.1186/gb-2011-12-1-r7
Karthik MVK, Satya Deepak MVKN, Shukla P (2012) Explication of interactions between HMGCR isoform 2 and various statins through In silico modeling and docking. Comp Biol Med 42:156–163. doi:10.1016/j.compbiomed.2011.11.003
Singh PK, Shukla P (2012) Molecular modeling and docking of microbial inulinases towards perceptive enzyme-substrate interactions. Ind J Microbiol 52:373–380. doi:10.1007/s12088-012-0248-0
Osborne CS, Leitner I, Favre B, Ryder NS (2005) Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother 49:2840–2844
Yang Z (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. doi:10.1186/1471-2105-9-40
Dong X, Yang Z (2011) Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J 101:2525–2534. doi:10.1016/j.bpj.2011.10.024
Bhattacharya A, Tejero R, Montelione GT (2007) Evaluating protein structures determined by structural genomics consortia. Proteins 66:778–795. doi:10.1002/prot.21165
Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008) DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucl Acids Res 36:D901–D906. doi:10.1093/nar/gkm958
Marcus DH, Donald EC, David CL, Tim V, Eva Z, Geoffrey RH (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:17. doi:10.1186/1758-2946-4-17
Lagorce D, Maupetit J, Baell J, Sperandio O, Tuffery P, Miteva MA, Galons H, Villoutreix BO (2011) The FAF-Drugs2 server: a multistep engine to prepare electronic chemical compound collections. Bioinformatics 27:2018–2020. doi:10.1093/bioinformatics/btr333
Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW (2010) HexServer: an FFT-based protein docking server powered by graphics processors. Nucl Acids Res 38:W445–W449. doi:10.1093/nar/gkq311
Dessalew N, Bharatam PV (2007) 3D-QSAR and molecular docking study on bisarylmaleimide series as glycogen synthase kinase 3, cyclin dependent kinase 2 and cyclin dependent kinase 4 inhibitors: an insight into the criteria for selectivity. Eur J Med Chem 42:1014–1027. doi:10.1016/j.ejmech.2007.01.010
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623. doi:10.1021/jm020017n
Steven B, Gilbert JB, Michael M (1978) Potential inhibitors of l-asparagine biosynthesis. 4. Substituted sulfonamide and sulfonylhydrazide analogs of l-asparagine. J Med Chem 978:45–49. doi:10.1021/jm00199a008
Acknowledgments
We hereby acknowledge BTIS-Sub DIC facility at BIT Mesra through DBT, GOI and the Department of Agriculture, Government of Jharkhand for financial support for our department towards infrastructure facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Sudha Karumuri and Puneet Kumar Singh should be regarded as the joint first authors.
Rights and permissions
About this article
Cite this article
Karumuri, S., Singh, P.K. & Shukla, P. In Silico Analog Design for Terbinafine Against Trichophyton rubrum: A Preliminary Study. Indian J Microbiol 55, 333–340 (2015). https://doi.org/10.1007/s12088-015-0524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-015-0524-x