Abstract
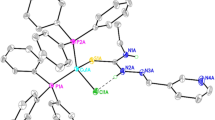
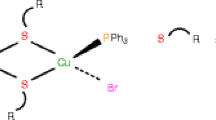
Thiazolidine-2-thione (L1, NC3H5S2) reacted with copper(I) bromide in CH3CN under aerobic conditions and transformed through C–S bond cleavage into 3-(2-thiazolin-2-yl)thiazolidine-2-thione (L2, C3H4S2N-C3H4SN). This thio-ligand L2 with CuI ion yielded a three coordinate complex, [3-(2-Thiazolin-2-yl)thiazolidine-2-thione]copper(I)bromide 1a which crystallized in the triclinic system with the space group P1 as reported earlier. Treatment of 1a with bis(diphenylphosphino)methane (dppm) in dichloromethane also formed [3-(2-thiazolin-2-yl)thiazolidine-2-thione]copper(I) bromide 1b but it crystallized into the triclinic system with a new space group, P-1: 296(2) K, a, 7.3890(19); b, 8.473(2); c, 9.491(2) Å; α, 70.273(5); β, 67.170(5); γ, 84.949(5)∘; R, 6.79%. Reactions of copper(II) nitrate with thiazolidine-2-thione followed by the addition of 2,2′-bipyridine or with 2,2′-bipyridine first followed by the addition of thiazolidine-2-thione, gave blue crystals in both the cases. The x-ray crystallography revealed stoichiometry of the complex formed as: [Cu(κ 2-N,N′-bipy)2(κ 1-ONO2)](NO3)2, which crystallized in monoclinic crystal system with space group, P21/n(14). Crystal data: 173(2) K, a, 11.318(1), b, 12.160(1), c, 14.967(1) Å; β = 98.01(1)∘, R, 3.99%; 296(2) K, a, 11.340(5), b, 12.249(5), c, 15.065(6) Å; β = 98.04(2)∘, R, 4.09%.

Thiazolidine-2-thione with copper(I) bromide in CH3CN under aerobic conditions yielded red brown crystals which on treatment with bis(diphenylphosphino)methane in dichloromethane formed [3-(2-thiazolin-2-yl)thiazolidine-2-thione]copper(I) bromide which crystallized into triclinic system with space group, P-1.






Similar content being viewed by others
References
T S Lobana, Razia Sultana, G Hundal and R J Butcher 2010 Dalton Trans. 39 7870
T S Lobana, Razia Sultana and R J Butcher 2011 Dalton Trans. 40 11382
T S Lobana, Razia Sultana, R J Butcher, A Castineiras, T Akitsu, F J Fernandez and M C Vega 2013 Eur. J. Inorg. Chem. 5161
A G Dodge, J E Richman, G Johnson and L P Wackett 2006 Appl. Environ. Microbiol. 72 7468
E W Ainscough, A G Bingham and A M Brodie 1985 Inorg. Chim. Acta 96 L47
J-K Cheng, Y-B Chen, L Wu, J Zhang, Y-H Wen, Z-J Li and Y-G Yao 2005 Inorg. Chem. 44 3386
(a) Liandi Wang, Wei He and Zhengkun Yu 2013 Chem. Soc. Rev. 42 599; (b) Hai-Bin Zhu and Shao-Hua Gou 2011 Coord. Chem. Rev. 255 318; (c) C Huang, S Gou, H Zhu and W Huang 2007 Inorg. Chem. 46 5537; (d) J Spencer, M Pfeffer, A DeCian and J Fischer 1995 J. Org. Chem. 60 1005; (e) K Pramanik, U Das, B Adhikari, D Chopra and H Stoeckli-Evans 2008 Inorg. Chem. 47 429; (f) H Kawaguchi, K Yamada, J P Lang and K Tatsumi 1997 J. Am. Chem. Soc. 119 10346
Oxford Diffraction 2009 CrysAlisPro CCD and CrysAlisPro RED Oxford Diffraction Ltd. Yarnton, England
G M Sheldrick 2008 Acta Crystallogr. Sect. A 64 112
A Altomare, G Cascarano, C Giacovazzo and A Guagliardi 1993 J. Appl. Crystallogr. 26 343
A J C Wilson 1995 In International Tables for Crystallography Vol. C (Netherlands: Kluwer Academic)
K Marjani, S C Davies, M C Durrant, D L Hughes, N Khodamorad and A Samodi 2005 Acta Crystallogr. Sect. E: Struct. Rep. Online 61 m11
P Y Zavalij, B L Burton and W E Jones Junior 2002 Acta Crystallogr. Sect.C: Cryst. Struct. Commun. 58 m330
M Moore, D A Knight, D Zabetakis, J R eschamps, W J Dressick, E L Chang, B Lascano, R Nita and S A Trammell 2012 Inorg. Chim. Acta 388 168
B L V Prasad, H Sato, T Enoki, S Cohen and T P Radhakrishnan 1999 J. Chem. Soc., Dalton Trans. 25
G A van Albada, A Mohamadou, I Mutikainen, U Turpeinen and J Reedijk 2004 Eur. J. Inorg. Chem. 3733
A S Potapov, G A Domina, T V Petrenko and A I Khlebnikov 2012 Polyhedron 33 150
R J Fereday, P Hodgson, S Tyagi and B J Hathaway 1981 J. Chem. Soc., Dalton Trans. 2070
K J Catalan, S Jackson, J D Zubkowski, D L Perry, E J Valente, L A Feliu and A Polanco 1995 Polyhedron 14 2165
H Nakai 1980 Bull. Chem. Soc. Jpn. 53 1321
R J Anderson, P H Hagback and P J Steel 1999 Inorg. Chim. Acta 284 273
M D Stephenson, T J Prior, M J Hardie 2008 Cryst. Growth Des. 8 643
Acknowledgements
Financial assistance in the form of Emeritus Scientist Grant [21(0904)/12-EMR-II] to T.S. Lobana, from the Council of Scientific and Industrial Research (CSIR), New Delhi, and from Department of Science and Technology (DST) for x-ray diffractometer grant to Department of Chemistry, GNDU, Amritsar are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK. Copies of the data can be obtained free of charge on quoting the depository numbers CCDC 1006272 for 1b, 988896 for 2a and 988897 for 2b (Fax: + 44-1223-336-033; E-Mail: deposit@ccdc.cam.ac.uk, http://www.ccdc.cam.ac.uk). (See Tables S1 and S2 for more details about nitrate coordination, complete bond lengths/angles for 2as well as for bonding trends of nitrate in presence of 2,2′-bipyridines).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
LOBANA, T.S., RANI, A., JASSAL, A.K. et al. Reactivity of thiazolidine-2-thione towards CuI/CuII: Synthesis and structures of [3-(2-thiazolin-2-yl)thiazolidine-2-thione]copper(I) bromide and [bis(2,2′-bipyridine)nitratocopper(II)] nitrate. J Chem Sci 127, 149–153 (2015). https://doi.org/10.1007/s12039-014-0760-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0760-3