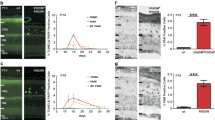
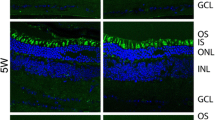
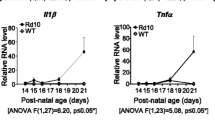
Abstract
Retinitis pigmentosa (RP) is a group of inherited neurological disorders characterized by rod photoreceptor cell death, followed by secondary cone cell death leading to progressive blindness. Currently, there are no viable treatment options for RP. Due to incomplete knowledge of the molecular signaling pathways associated with RP pathogenesis, designing therapeutic strategies remains a challenge. In particular, preventing secondary cone photoreceptor cell loss is a key goal in designing potential therapies. In this study, we identified the main drivers of rod cell death and secondary cone loss in the transgenic S334ter rhodopsin rat model, tested the efficacy of specific cell death inhibitors on retinal function, and compared the effect of combining drugs to target multiple pathways in the S334ter and P23H rhodopsin rat models. The primary driver of early rod cell death in the S334ter model was a caspase-dependent process, whereas cone cell death occurred though RIP3-dependent necroptosis. In comparison, rod cell death in the P23H model was via necroptotic signaling, whereas cone cell loss occurred through inflammasome activation. Combination therapy of four drugs worked better than the individual drugs in the P23H model but not in the S334ter model. These differences imply that treatment modalities need to be tailored for each genotype. Taken together, our data demonstrate that rationally designed genotype-specific drug combinations will be an important requisite to effectively target primary rod cell loss and more importantly secondary cone survival.








Similar content being viewed by others
References
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Berson EL (1993) Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34:1659–1676
Sohocki MM, Daiger SP, Browne SJ, Rodriguez H, Northrup JR, Heckenlively DG et al (2001) Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 17:42–51
Ripps H (2002) Cell death in retinitis pigmentosa: gap junctions and the 'bystander' effect. Exp Eye Res 74:327–336
Gupta N, Brown KE, Milam AH (2003) Activated microglia in human retinitis pigmentosa, late onset retinal degeneration, and age-related macular degeneration. Exp Eye Res 76:463–471
Komeima K, Rogers BS, Campochiaro PA (2007) Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol 213:809–815
Punzo C, Kornacker K, Cepko CL (2009) Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12:44–52
Byrne LC, Dalkara D, Luna G, Fisher SK, Clerin E, Sahal JA et al (2015) Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Invest 125:105–116
Viringipurampeer IA, Metcalfe AL, Bashar AE, Sivak O, Yanai Y, Mohammadi Z et al (2016) NLRP3 inflammasome activation drives bystander cone photoreceptor cell death in a P23H rhodopsin model of retinal degeneration. Hum Mol Genet 25:1501–1516
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K et al (2008) Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358:2231–2239
Birch DG, Bennett LD, Duncan JL, Weleber RG, Pennesi ME (2016) Long-term follow-up of patients with retinitis pigmentosa receiving intraocular ciliary neurotrophic factor implants. Am J Ophthalmol 170:10–14
Osborne NN (2009) Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol 87:450–454
Tawbi H, Nimmagadda N (2009) Targeted therapy in melanoma. Biologics 3:475–484
Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT et al (1997) Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 337:734–739
Reboldi G, Gentile G, Angeli F, Verdecchia P (2009) Choice of ACE inhibitor combinations in hypertensive patients with type 2 diabetes: update after recent clinical trials. Vasc Health Risk Manag 5:411–427
Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R, CV181-039 Investigators (2009) Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 11:611–622
Cheng JW, Li Y, Wei RL (2009) Systematic review of intraocular pressure-lowering effects of adjunctive medications added to latanoprost. Ophthalmic Res 42:99–105
Agarwal M, Ganesh SK, Biswas J (2006) Triple agent immunosuppressive therapy in Vogt-Koyanagi-Harada syndrome. Ocul Immunol Inflamm 14:333–339
Saliba RS, Munro PMG, Luthert PJ, Cheetham ME (2002) The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci 15:2907–2918
Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, Kaushal S (2003) Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem 278:14442–14450
Oh KT, Longmuir R, Oh DM, Stone EM, Kopp K, Brown J, Fishman GA, Sonkin P et al (2003) Comparison of the clinical expression of retinitis pigmentosa associated with rhodopsin mutations at codon 347 and codon 23. Am J Ophthalmol 136:306–313
Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW et al (1990) A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 343:364–366
Green ES, Menz MD, LaVail MM, Flannery JG (2000) Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:1546–1553
Sandberg MA, Weigel-DiFranco C, Dryja TP, Berson EL (1995) Clinical expression correlates with location of rhodopsin mutation in dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 36:1934–1942
Wasowicz M, Morice C, Ferrari P, Callebert J, Versaux-Botteri C (2002) Long-term effects of light damage on the retina of albino and pigmented rats. Invest Ophthalmol Vis Sci 43:813–820
Gregory-Evans K, Po K, Chang F, Gregory-Evans CY (2012) Pharmacological enhancement of ex vivo gene therapy neuroprotection in a rodent model of retinal degeneration. Ophthalmic Res 47:32–38
Kaur J, Mencl S, Sahaboglu A, Farinelli P, van Veen T, Ekström P et al (2011) Calpain and PARP activation during photoreceptor cell death in P23H and S334ter rhodopsin mutant rats. PLoS One 6:e22181
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Brown GC, Neher JJ (2012) Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis. Trends Biochem Sci 37:325–332
Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN et al (2015) Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med 7:1179–1197
Liu C, Li Y, Peng M, Laties AM, Wen R (1999) Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J Neurosci 19:4778–4785
Arango-Gonzalez B, Trifunov D, Sahaboglu A, Michalakis S, Farinelli P, Koch F et al (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9:e112142
Murakami Y, Matsumoto H, Roh M, Suzuki J, Hisatomi Y, Ikeda Y et al (2012) Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc Natl Acad Sci U S A 109:14598–14603
Sato K, Li S, Gordon WC, He J, Liou GI, Hill JM, Travis GH, Bazan NG et al (2013) Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein. J Neurosci 33:17458–17468
Viringipurampeer IA, Shan X, Gregory-Evans K, Zhang JP, Mohammadi Z, Gregory-Evans CY (2014) Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish. Cell Death Diff 21:665–675
Murakami Y, Ikeda Y, Yonemitsu Y, Onimaru M, Nakagawa K, Kohno R, Miyazaki M, Hisatomi T et al (2008) Inhibition of nuclear translocation of apoptosis-inducing factor is an essential mechanism of neuroprotective activity of pigment epithelium-derived factor in a rat model of retinal degeneration. Am J Pathol 173:1326–1338
Zhang T, Wei Y, Jiang X, Li J, Qui S, Zhang S (2015) Protection of photoreceptors by intravitreal injection of the Y-27632 Rho-associated protein kinase inhibitor in Royal College of Surgeons rats. Mol Med Rep 12:3655–3661
Sanges D, Comitato A, Tammaro R, Marigo V (2006) Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci U S A 103:17366–17371
Boatright JH, Moring AG, McElroy C, Phillips MJ, Do VT, Chang B, Hawes NL, Boyd AP et al (2006) Tool from ancient pharmacopoeia prevents vision loss. Mol Vis 12:1706–1714
Lawson EC, Bhatia SK, Han MK, Aung MH, Ciavatta V, Boatright JH et al (2016) Tauroursodeoxycholic acid protects retinal function and structure in rd1 mice. Adv Exp Med Biol 854:431–436
Clemson CM, Tzekov R, Krebs M, Checchi JM, Bigelow C, Kaushal S (2011) Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol 95:89–93
Falsini B, Larossi G, Chiaretti A, Ruggiero A, Luigi M, Galli-Resta L et al (2016) NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J Transl Med 14:8
Scholl HP, Moore AT, Koenekoop RK, Wen Y, Fishman GA, van den Born LI et al (2015) Safety and proof-of-concept study of oral QLT091001 in retinitis pigmentosa due to inherited deficiencies of retinal pigment epithelial 65 protein (RPE65) or lecithin:retinol acyltransferase (LRAT). PLoS One 10:e0143846
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471:368–372
Kovalenko A, Kim J-C, Kang T-B, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O et al (2009) Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med 206:2161–2177
Orhan E, Dalkara D, Neuille M, Lechauve C, Michiels C, Picaud S et al (2015) Genotypic and phenotypic characterization of P23H line 1 rat model. PLoS One 10:e0127319
Sakima S, Maeda T, Bereta G, Okano K, Golczak M, Sumaroka A et al (2011) Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J Biol Chem 286:10551–10567
Chen J, Makino CL, Peachey NS, Baylor DA, Simon MI (1995) Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science 267:374–377
Sung C-H, Makino C, Baylor D, Nathans J (1994) A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci 14:5818–5833
Hollingsworth TJ, Gross AK (2013) The severe autosomal dominant retinitis pigmentosa rhodopsin mutant Ter349Glu mislocalizes and induces rapid rod cell death. J Biol Chem 288:29047–29055
Sung C-H, Davenport CM, Hennessey JC, Maumenee IH, Jacobson SG, Heckenlively JR, et al (1991) Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A 88:648l-6485
Thanos S (1992) Sick photoreceptors attract activated microglia from the ganglion cell layer: a model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res 588:21–28
Noailles A, Fernández-Sánchez L, Lax P, Cuenca N (2014) Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J Neuroinflamm 29:176–186
Karlsetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T (2015) Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45:30–57
Peng B, Xiao J, Wang K, So KF, Tipoe GL, Lin B (2014) Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci 34:8139–3150
Zeng H, Ding M, Chen XX, Lu Q (2014) Microglial NADPH oxidase activation mediates rod cell death in the retinal degeneration in rd mice. Neuroscience 275:54–61
Hughes EH, Schlichtenbrede FC, Murphy CC, Broderick C, van Rooijen N, Ali RR, Dick AD (2004) Minocycline delays photoreceptor death in the rds mouse through a microglia-independent mechanism. Exp Eye Res 78:1077–1084
Hilla AM, Diekmann H, Fischer D (2017) Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci 37:6113–6124
Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO (2004) Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci 45:2753–2759
Kohno H, Chen Y, Kevany BM, Pearlman E, Miyagi M, Maeda T, Palczewski K, Maeda A (2013) Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem 288:15326–15341
Anderson RE, Maude MB, McClellan M, Matthes MT, Yasumura D, LaVail MM (2002) Low docosahexaenoic acid levels in rod outer segments of rats with P23H and S334ter rhodopsin mutations. Mol Vis 8:351–358
Mirza M, Volz C, Karlsetter M, Langiu M, Somogyi A, Ruonala MO et al (2013) Progressive retinal degeneration and glial activation in the CLN6 (nclf) mouse model of neuronal ceroid lipofuscinosis: a beneficial effect of DHA and curcumin supplementation. PLoS One 8:e75963
Vasireddy V, Chavali VR, Joseph VT, Kadam R, Lin JH, Jamison JA et al (2011) Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS One 6:e21193
Acknowledgements
This work was supported by the Canadian Institutes of Health Research Team Grant (grant number 222728 to KGE, CGE, and OLM). The authors acknowledge the use of the Wax-it Histology Service Facility, the Rodent Functional Testing Suite, and Dr. A. Gharhary at iCORD for access to the Real-Time PCR facility, all at the University of British Columbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare there is no conflict of interest in the current study.
Rights and permissions
About this article
Cite this article
Viringipurampeer, I.A., Gregory-Evans, C.Y., Metcalfe, A.L. et al. Cell Death Pathways in Mutant Rhodopsin Rat Models Identifies Genotype-Specific Targets Controlling Retinal Degeneration. Mol Neurobiol 56, 1637–1652 (2019). https://doi.org/10.1007/s12035-018-1192-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1192-8