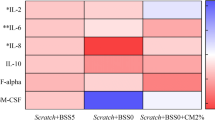
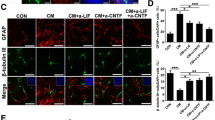
Abstract
Previous studies have indicated that paracrine factors (conditioned medium) increase wound closure and reduce reactive oxygen species in a traumatic brain injury in vitro model. Although the beneficial effects of conditioned medium from human adipose tissue-derived mesenchymal stem cells (hMSCA-CM) have been previously suggested for various neurological diseases, their actions on astrocytic cells are not well understood. In this study, we have explored the effect of hMSCA-CM on human astrocyte model (T98G cells) subjected to scratch assay. Our results indicated that hMSCA-CM improved cell viability, reduced nuclear fragmentation, attenuated the production of reactive oxygen species, and preserved mitochondrial membrane potential and ultrastructural parameters. In addition, hMSCA-CM upregulated neuroglobin in T98G cells and the genetic silencing of this protein prevented the protective action of hMSCA-CM on damaged cells, suggesting that neuroglobin is mediating, at least in part, the protective effect of hMSCA-CM. Overall, this evidence suggests that the use of hMSCA-CM is a promising therapeutic strategy for the protection of astrocytic cells in central nervous system (CNS) pathologies.








Similar content being viewed by others
References
Wei X et al (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34(6):747–754
Toma C et al (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105(1):93–98
Guerit D et al (2014) FOXO3A regulation by miRNA-29a controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem Cells Dev 23(11):1195–1205
Oswald J et al (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22(3):377–384
Taniguchi H et al. (2016) An Efficient Method to Obtain Dedifferentiated Fat Cells. J Vis Exp (113)
Gnecchi M et al (2016) Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol 1416:123–146
Huang B et al (2016) Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med 14:45
Hwang B et al (2016) Pretreatment with bone marrow-derived mesenchymal stromal cell-conditioned media confers pulmonary ischemic tolerance. J Thorac Cardiovasc Surg 151(3):841–846
Lee JC et al (2016) Notice of retraction: therapeutic effects of umbilical cord blood derived mesenchymal stem cell-conditioned medium on pulmonary arterial hypertension in rats. J Pathol Transl Med 50(4):325
Pires AO et al (2016) Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev 25(14):1073–1083
Pischiutta F et al (2016) Protection of brain injury by amniotic mesenchymal stromal cell-secreted metabolites. Crit Care Med 44(11):e1118–e1131
Kupcova Skalnikova H (2013) Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 95(12):2196–2211
Kilroy GE et al (2007) Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 212(3):702–709
Linero I, Chaparro O (2014) Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One 9(9):e107001
Hao P et al (2014) Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab Brain Dis 29(1):193–205
Wei X et al (2009) IFATS collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 27(2):478–488
Cirillo G et al (2011) Reactive astrocytosis-induced perturbation of synaptic homeostasis is restored by nerve growth factor. Neurobiol Dis 41(3):630–639
Deng LX et al (2011) GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol 229(2):238–250
Mita T et al (2015) Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer's disease. Behav Brain Res 293:189–197
Guo ZY et al (2015) Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res 10(4):651–658
Song M et al (2015) Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J Neurosci Res 93(6):973–983
Torrente D et al (2014) Paracrine factors of human mesenchymal stem cells increase wound closure and reduce reactive oxygen species production in a traumatic brain injury in vitro model. Hum Exp Toxicol 33(7):673–684
Huang W et al (2015) Paracrine factors secreted by MSCs promote astrocyte survival associated with GFAP downregulation after ischemic stroke via p38 MAPK and JNK. J Cell Physiol 230(10):2461–2475
Sun H et al (2013) Therapeutic potential of mesenchymal stromal cells and MSC conditioned medium in amyotrophic lateral sclerosis (ALS)—in vitro evidence from primary motor neuron cultures, NSC-34 cells, astrocytes and microglia. PLoS One 8(9):e72926
Salgado AJ et al (2010) Role of human umbilical cord mesenchymal progenitors conditioned media in neuronal/glial cell densities, viability, and proliferation. Stem Cells Dev 19(7):1067–1074
Cho YJ et al (2012) Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res 90(9):1794–1802
Egashira Y et al (2012) The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res 1461:87–95
Yamazaki H et al (2015) Adipose-derived stem cell-conditioned medium ameliorates antidepression-related behaviors in the mouse model of Alzheimer's disease. Neurosci Lett 609:53–57
Yousefi F et al (2016) In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis. Immunol Lett 172:94–105
Baez E et al (2016) Protection by neuroglobin expression in brain pathologies. Front Neurol 7:146
Avivi A et al (2010) Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proc Natl Acad Sci U S A 107(50):21570–21575
Lechauve C et al (2013) Neuroglobin involvement in visual pathways through the optic nerve. Biochim Biophys Acta 1834(9):1772–1778
Avila-Rodriguez M et al (2016) Tibolone protects astrocytic cells from glucose deprivation through a mechanism involving estrogen receptor beta and the upregulation of neuroglobin expression. Mol Cell Endocrinol 433:35–46
Yu ZL et al (2014) Neuroglobin—a potential biological marker of retinal damage induced by LED light. Neuroscience 270:158–167
Acaz-Fonseca E et al. (2015) Sex differences in glia reactivity after cortical brain injury. Glia
Yu Z et al (2016) Roles of neuroglobin binding to mitochondrial complex III subunit cytochrome c1 in oxygen-glucose deprivation-induced neurotoxicity in primary neurons. Mol Neurobiol 53(5):3249–3257
Taylor JM et al (2014) Neuroglobin overexpression improves sensorimotor outcomes in a mouse model of traumatic brain injury. Neurosci Lett 577:125–129
Yu Z et al (2012) Neuroglobin, a novel target for endogenous neuroprotection against stroke and neurodegenerative disorders. Int J Mol Sci 13(6):6995–7014
Zhao S et al (2012) Neuroglobin-overexpression reduces traumatic brain lesion size in mice. BMC Neurosci 13:67
Zhou Z et al (2013) Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 15(4):434–448
Avila Rodriguez M et al (2014) Tibolone protects T98G cells from glucose deprivation. J Steroid Biochem Mol Biol 144 Pt B:294–303
Cabezas R et al (2015) PDGF-BB protects mitochondria from rotenone in T98G cells. Neurotox Res 27(4):355–367
Mimura J et al (2011) Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J Biochem 150(2):209–217
Sasaki S et al (2015) Functional characterization of 5-oxoproline transport via SLC16A1/MCT1. J Biol Chem 290(4):2303–2311
Toro-Urrego N et al (2016) Testosterone protects mitochondrial function and regulates neuroglobin expression in astrocytic cells exposed to glucose deprivation. Front Aging Neurosci 8:152
Bourguignon LY et al (2007) Hyaluronan-CD44 interaction stimulates Rac1 signaling and PKN gamma kinase activation leading to cytoskeleton function and cell migration in astrocytes. J Neurochem 101(4):1002–1017
Ouyang Y-B et al (2011) Overexpressing GRP78 influences Ca 2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 11(2):279–286
Oliva CR et al (2011) Acquisition of chemoresistance in gliomas is associated with increased mitochondrial coupling and decreased ROS production. PLoS One 6(9):e24665
Jeong SH et al (2014) Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar Drugs 12(8):4602–4615
Habersetzer J et al (2013) Human F1F0 ATP synthase, mitochondrial ultrastructure and OXPHOS impairment: a (super-)complex matter? PLoS One 8(10):e75429
Janer A et al (2016) SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol Med 8(9):1019–1038
Ott C et al (2012) Sam50 functions in mitochondrial intermembrane space bridging and biogenesis of respiratory complexes. Mol Cell Biol 32(6):1173–1188
Birk AV et al (2013) The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24(8):1250–1261
Bankhead P (2013) Analyzing fluorescence microscopy images with ImageJ. Nikon Imaging Center, Heidelberg University, Queen’s University Belfast
Collard JF, Cote F, Julien JP (1995) Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature 375(6526):61–64
Bertheuil N et al (2015) Adipose-derived stromal cells: history, isolation, immunomodulatory properties and clinical perspectives. Ann Chir Plast Esthet 60(2):94–102
Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95(1):9–20
Gnecchi M et al (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103(11):1204–1219
Mirotsou M et al (2011) Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 50(2):280–289
Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 3:359
Liang CM et al (2014) Neurotrophic and neuroprotective potential of human limbus-derived mesenchymal stromal cells. Cytotherapy 16(10):1371–1383
Scheibe F et al (2012) Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol Neurobiol 32(4):567–576
Chen D, et al. (2016) Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson's disease. J Neurosci Res
Xie B et al (2016) Therapeutic effects of human umbilical cord mesenchymal stem cells transplantation on hypoxic ischemic encephalopathy. Am J Transl Res 8(7):3241–3250
Allen J, Knoblach S, Faden A (1999) Combined mechanical trauma and metabolic impairment in vitro induces NMDA receptor-dependent neuronal cell death and caspase-3-dependent apoptosis. FASEB J 13(13):1875–1882
Greve MW, Zink BJ (2009) Pathophysiology of traumatic brain injury. Mt Sinai J Med 76(2):97–104
Kallakuri S et al (2015) Traumatic brain injury by a closed head injury device induces cerebral blood flow changes and Microhemorrhages. J Clin Imaging Sci 5:52
Prins M et al (2013) The pathophysiology of traumatic brain injury at a glance. Dis Model Mech 6(6):1307–1315
Lattanzi W et al (2011) Neurotrophic features of human adipose tissue-derived stromal cells: in vitro and in vivo studies. J Biomed Biotechnol 2011:468705
Ong WK, Sugii S (2013) Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol 45(6):1083–1086
Cantinieaux D et al (2013) Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One 8(8):e69515
Anne Stetler R et al (2013) The dynamics of the mitochondrial organelle as a potential therapeutic target. J Cereb Blood Flow Metab 33(1):22–32
Duchen MR, Szabadkai G (2010) Roles of mitochondria in human disease. Essays Biochem 47:115–137
Picard M et al (2013) Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol 304(6):R393–R406
Nakashima-Kamimura N et al (2005) MIDAS/GPP34, a nuclear gene product, regulates total mitochondrial mass in response to mitochondrial dysfunction. J Cell Sci 118(Pt 22):5357–5367
Kaewsuya P, Danielson ND, Ekhterae D (2007) Fluorescent determination of cardiolipin using 10-N-nonyl acridine orange. Anal Bioanal Chem 387(8):2775–2782
Witte I et al (2011) Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells. Cell Death Dis 2:e112
Yuan Y et al (2016) Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1alpha pathway. Clin Sci (Lond) 130(23):2181–2198
Kemp K et al (2010) Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem 114(6):1569–1580
Lin WP et al (2013) Effect of neuroglobin genetically modified bone marrow mesenchymal stem cells transplantation on spinal cord injury in rabbits. PLoS One 8(5):e63444
Platas J et al (2016) Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes. Aging (Albany NY) 8(8):1703–1717
van Koppen A et al (2012) Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One 7(6):e38746
Fernandez-Fernandez S, Almeida A, Bolanos JP (2012) Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J 443(1):3–11
Griffiths CE, Voorhes JJ (1990) Cyclosporine A in the treatment of psoriasis: a clinical and mechanistic perspective. J Invest Dermatol 95(5):53S–55S
Wilson JX (1997) Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol 75(10–11):1149–1163
Kim WS et al (2008) Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 49(2):133–142
Yalvac ME et al (2013) Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro-environment. Brain Behav Immun 32:122–130
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130
Pekny M, Pekna M (2004) Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol 204(4):428–437
Wilhelmsson U et al (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 24(21):5016–5021
George E, Barreto JG, Capani F and Morales L (2011) Role of astrocytes in neurodegenerative diseases, in neurodegenerative diseases—processes, prevention, protection and monitoring
Shafit-Zagardo B, Kume-Iwaki A, Goldman JE (1988) Astrocytes regulate GFAP mRNA levels by cyclic AMP and protein kinase C-dependent mechanisms. Glia 1(5):346–354
Banasr M et al (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15(5):501–511
Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M (1999) Intermediate filament protein partnership in astrocytes. J Biol Chem 274:23996–24006
Hue L (1976) The use of [14C,3H] glucose in the study of so-called 'futile cycles' in liver. Biochem Soc Trans 4(6):994–998
Lee HH et al (2015) Time course and characteristics of astrocyte activation in the rat brain after injury. Korean J Neurotrauma 11(2):44–51
Nunnari J, Suomalainen A (2012) Mitochondria: in sickness and in health. Cell 148(6):1145–1159
Zick M, Rabl R, Reichert AS (2009) Cristae formation-linking ultrastructure and function of mitochondria. Biochim Biophys Acta 1793(1):5–19
Rampelt H, et al. (2016) Role of the mitochondrial contact site and cristae organizing system in membrane architecture and dynamics. Biochim Biophys Acta
Cogliati S, Enriquez JA, Scorrano L (2016) Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci 41(3):261–273
Salgado AJ et al (2010) Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 5(2):103–110
Walter MN et al (2010) Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 316(7):1271–1281
Aizman I, McGrogan M, Case CC (2013) Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis. Stem Cells Transl Med 2(3):223–232
Hu L et al (2016) Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 6:32993
Yuan H, et al. (2016) Exosomes secreted by human urine-derived stem cells accelerate skin wound healing by promoting angiogenesis in rat. Cell Biol Int
Qi X et al (2016) Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci 12(7):836–849
Zhang J et al (2015) Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med 13:49
Eirin A et al (2014) MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 551(1):55–64
Pegtel DM et al (2010) Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 107(14):6328–6333
Liu Y et al (2013) Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One 8(11):e78514
Park CM et al (2016) Umbilical cord mesenchymal stem cell-conditioned media prevent muscle atrophy by suppressing muscle atrophy-related proteins and ROS generation. In Vitro Cell Dev Biol Anim 52(1):68–76
Li L et al (2014) Neuroglobin promotes neurite outgrowth via differential binding to PTEN and Akt. Mol Neurobiol 49(1):149–162
Li Y et al (2016) Neuroglobin attenuates beta amyloid-induced apoptosis through inhibiting caspases activity by activating PI3K/Akt signaling pathway. J Mol Neurosci 58(1):28–38
Sandhir R (2014) Neuroglobin increases brain fitness: commentary to: "Neuroglobin overexpression improves sensorimotor outcomes in a mouse model of traumatic brain injury". Neurosci Lett 577:123–124
Liu Y et al (2015) Neuroglobin up-regulation after ischaemic pre-conditioning in a rat model of middle cerebral artery occlusion. Brain Inj 29(5):651–657
Ren C et al (2015) Limb remote ischemic per-conditioning in combination with post-conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci 33(3):369–379
Jin K et al (2010) Neuroglobin expression in ischemic stroke. Stroke 41(3):557–559
Li Q et al (2004) A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24(16):4070–4081
Fiocchetti M et al (2015) ERbeta-dependent neuroglobin up-regulation impairs 17beta-estradiol-induced apoptosis in DLD-1 colon cancer cells upon oxidative stress injury. J Steroid Biochem Mol Biol 149:128–137
De Marinis E et al (2013) 17beta-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor beta-mediated neuroglobin up-regulation. J Neuroendocrinol 25(3):260–270
Chen X et al (2015) Long-term neuroglobin expression of human astrocytes following brain trauma. Neurosci Lett 606:194–199
Yu Z et al (2012) Mitochondrial distribution of neuroglobin and its response to oxygen-glucose deprivation in primary-cultured mouse cortical neurons. Neuroscience 218:235–242
Oliveira KC et al (2015) Thyroid hormone modulates neuroglobin and cytoglobin in rat brain. Metab Brain Dis 30(6):1401–1408
Amri F et al (2017) Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J Neurochem 140(1):151–169
Gonzalez A, Pariente JA, Salido GM (2007) Ethanol stimulates ROS generation by mitochondria through Ca2+ mobilization and increases GFAP content in rat hippocampal astrocytes. Brain Res 1178:28–37
Acknowledgments
The authors thank Dr. Jorge Andres Afanador and the staff of the cosmetic surgery Clinic DHARA in Bogotá - Colombia, for the adipose tissue samples. This work was supported by PUJ grant #6260 to GEB and scholarship for doctoral studies awarded by the Vicerrectoria Académica of PUJ to Baez-Jurado E.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baez-Jurado, E., Vega, G.G., Aliev, G. et al. Blockade of Neuroglobin Reduces Protection of Conditioned Medium from Human Mesenchymal Stem Cells in Human Astrocyte Model (T98G) Under a Scratch Assay. Mol Neurobiol 55, 2285–2300 (2018). https://doi.org/10.1007/s12035-017-0481-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0481-y