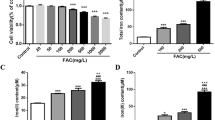
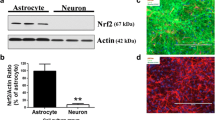
Abstract
This study was aimed at evaluating the role of increased iron accumulation in oligodendrocytes and its role in their apoptosis in the periventricular white matter damage (PWMD) following a hypoxic injury to the neonatal brain. In response to hypoxia, in the PWM, there was increased expression of proteins involved in iron acquisition, such as iron regulatory proteins (IRP1, IRP2) and transferrin receptor in oligodendrocytes. Consistent with this, following a hypoxic exposure, there was increased accumulation of iron in primary cultured oligodendrocytes. The increased concentration of iron within hypoxic oligodendrocytes was found to elicit ryanodine receptor (RyR) expression, and the expression of endoplasmic reticulum (ER) stress markers such as binding-immunoglobulin protein (BiP) and inositol-requiring enzyme (IRE)-1α. Associated with ER stress, there was reduced adenosine triphosphate (ATP) levels within hypoxic oligodendrocytes. However, treatment with deferoxamine reduced the increased expression of RyR, BiP, and IRE-1α and increased ATP levels in hypoxic oligodendrocytes. Parallel to ER stress there was enhanced reactive oxygen species production within mitochondria of hypoxic oligodendrocytes, which was attenuated when these cells were treated with deferoxamine. At the ultrastructural level, hypoxic oligodendrocytes frequently showed dilated ER and disrupted mitochondria, which became less evident in those treated with deferoxamine. Associated with these subcellular changes, the apoptosis of hypoxic oligodendrocytes was evident with an increase in p53 and caspase-3 expression, which was attenuated when these cells were treated with deferoxamine. Thus, the present study emphasizes that the excess iron accumulated within oligodendrocytes in hypoxic PWM could result in their death by eliciting ER stress and mitochondrial disruption.






Similar content being viewed by others
References
Beard JL, Connor JR (2003) Iron status and neural functioning. Annu Rev Nutr 23:41–58. doi:10.1146/annurev.nutr.23.020102.075739
Connor JR, Menzies SL (1996) Relationship of iron to oligodendrocytes and myelination. Glia 17(2):83–93. doi:10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7
Beard J (2003) Iron deficiency alters brain development and functioning. J Nutr 133(5 Suppl 1):1468S–1472S
Lozoff B, Georgieff MK (2006) Iron deficiency and brain development. Semin Pediatr Neurol 13(3):158–165. doi:10.1016/j.spen.2006.08.004
Pinero DJ, Connor JR (2000) Iron in the brain: an important contributor in normal and diseased states. Neuroscientist 6(6):435–453
Rathnasamy G, Ling EA, Kaur C (2011) Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species. J Neurosci 31(49):17982–17995. doi:10.1523/jneurosci.2250-11.2011
Alvarez-Diaz A, Hilario E, de Cerio FG, Valls-i-Soler A, Alvarez-Diaz FJ (2007) Hypoxic-ischemic injury in the immature brain—key vascular and cellular players. Neonatology 92(4):227–235. doi:10.1159/000103741
Baud O, Daire JL, Dalmaz Y, Fontaine RH, Krueger RC, Sebag G, Evrard P, Gressens P et al (2004) Gestational hypoxia induces white matter damage in neonatal rats: a new model of periventricular leukomalacia. Brain Pathol 14(1):1–10
Huang BY, Castillo M (2008) Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics 28(2):417–439. doi:10.1148/rg.282075066
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21(4):1302–1312
Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM (2002) Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22(2):455–463
Benkovic SA, Connor JR (1993) Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J Comp Neurol 338(1):97–113
Connor JR, Pavlick G, Karli D, Menzies SL, Palmer C (1995) A histochemical study of iron-positive cells in the developing rat brain. J Comp Neurol 355(1):111–123. doi:10.1002/cne.903550112
Yu GS, Steinkirchner TM, Rao GA, Larkin EC (1986) Effect of prenatal iron deficiency on myelination in rat pups. Am J Pathol 125(3):620–624
Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, Connor JR (2004) Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 77(5):681–689. doi:10.1002/jnr.20207
Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR (2009) Oligodendrocytes and myelination: the role of iron. Glia 57(5):467–478
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67(3):1014–1022
Juurlink BH (1997) Response of glial cells to ischemia: roles of reactive oxygen species and glutathione. Neurosci Biobehav Rev 21(2):151–166
Hemdan S, Almazan G (2007) Deficient peroxide detoxification underlies the susceptibility of oligodendrocyte progenitors to dopamine toxicity. Neuropharmacology 52(6):1385–1395. doi:10.1016/j.neuropharm.2007.01.019
Winterbourn CC (1995) Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 82–83:969–974
Youdim MB, Ben-Shachar D, Yehuda S, Riederer P (1990) The role of iron in the basal ganglion. Adv Neurol 53:155–162
Wardman P, Candeias LP (1996) Fenton chemistry: an introduction. Radiat Res 145(5):523–531
Rouault TA, Hentze MW, Haile DJ, Harford JB, Klausner RD (1989) The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci U S A 86(15):5768–5772
Eisenstein RS (2000) Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr 20:627–662. doi:10.1146/annurev.nutr.20.1.627
Schneider BD, Leibold EA (2003) Effects of iron regulatory protein regulation on iron homeostasis during hypoxia. Blood 102(9):3404–3411. doi:10.1182/blood-2003-02-0433
Toth I, Yuan L, Rogers JT, Boyce H, Bridges KR (1999) Hypoxia alters iron-regulatory protein-1 binding capacity and modulates cellular iron homeostasis in human hepatoma and erythroleukemia cells. J Biol Chem 274(7):4467–4473
Rao RV, Ellerby HM, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11(4):372–380. doi:10.1038/sj.cdd.4401378
Yoshida H (2007) ER stress and diseases. FEBS J 274(3):630–658. doi:10.1111/j.1742-4658.2007.05639.x
Malhotra JD, Kaufman RJ (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9(12):2277–2293. doi:10.1089/ars.2007.1782
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529. doi:10.1038/nrm2199
Bodalia A, Li H, Jackson MF (2013) Loss of endoplasmic reticulum Ca2+ homeostasis: contribution to neuronal cell death during cerebral ischemia. Acta Pharmacol Sin 34(1):49–59. doi:10.1038/aps.2012.139
Inagi R (2010) Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 10(2):156–165. doi:10.1016/j.coph.2009.11.006
Park EJ, Choi DH, Kim Y, Lee EW, Song J, Cho MH, Kim JH, Kim SW (2014) Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and ER stress in RAW264.7 cells. Toxicol In Vitro 28(8):1402–1412. doi:10.1016/j.tiv.2014.07.010
Cano M, Wang L, Wan J, Barnett BP, Ebrahimi K, Qian J, Handa JT (2014) Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic Biol Med 69:1–14. doi:10.1016/j.freeradbiomed.2014.01.004
Sheldon RA, Chuai J, Ferriero DM (1996) A rat model for hypoxic-ischemic brain damage in very premature infants. Biol Neonate 69(5):327–341
Giulian D, Baker TJ (1986) Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6(8):2163–2178
Saura J, Tusell JM, Serratosa J (2003) High-yield isolation of murine microglia by mild trypsinization. Glia 44(3):183–189. doi:10.1002/glia.10274
McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85(3):890–902
Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM (1996) Expression of the APC tumor suppressor protein in oligodendroglia. Glia 17(2):169–174. doi:10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y
Lang J, Maeda Y, Bannerman P, Xu J, Horiuchi M, Pleasure D, Guo F (2013) Adenomatous polyposis coli regulates oligodendroglial development. J Neurosci 33(7):3113–3130. doi:10.1523/jneurosci.3467-12.2013
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW (2001) Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci 23(3):203–208
Murugan M, Sivakumar V, Lu J, Ling EA, Kaur C (2011) Expression of N-methyl D-aspartate receptor subunits in amoeboid microglia mediates production of nitric oxide via NF-kappaB signaling pathway and oligodendrocyte cell death in hypoxic postnatal rats. Glia 59(4):521–539. doi:10.1002/glia.21121
Dugan LL, Choi DW (1994) Excitotoxicity, free radicals, and cell membrane changes. Ann Neurol 35(Suppl):S17–21
Youdim MB, Ben-Shachar D, Riederer P (1993) The possible role of iron in the etiopathology of Parkinson’s disease. Mov Disord: Off J Mov Disord Soc 8(1):1–12. doi:10.1002/mds.870080102
Muhoberac BB, Vidal R (2013) Abnormal iron homeostasis and neurodegeneration. Front Aging Neurosci 5:32. doi:10.3389/fnagi.2013.00032
Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, Drexler IR, Chen X et al (2009) Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell 16(1):35–46. doi:10.1016/j.devcel.2008.12.002
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119(1):37–53. doi:10.1007/s00401-009-0601-5
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115(10):2656–2664. doi:10.1172/jci26373
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190. doi:10.1038/ncb0311-184
Hajnoczky G, Csordas G, Madesh M, Pacher P (2000) Control of apoptosis by IP(3) and ryanodine receptor driven calcium signals. Cell Calcium 28(5-6):349–363. doi:10.1054/ceca.2000.0169
Ruiz A, Matute C, Alberdi E (2010) Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis 1:e54. doi:10.1038/cddis.2010.31
Wang H, Dong Y, Zhang J, Xu Z, Wang G, Swain CA, Zhang Y, Xie Z (2014) Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br J Anaesth 113(4):695–707. doi:10.1093/bja/aeu053
Kawakami M, Okabe E (1998) Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol Pharmacol 53(3):497–503
Munoz P, Zavala G, Castillo K, Aguirre P, Hidalgo C, Nunez MT (2006) Effect of iron on the activation of the MAPK/ERK pathway in PC12 neuroblastoma cells. Biol Res 39(1):189–190
Gething MJ (1999) Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol 10(5):465–472. doi:10.1006/scdb.1999.0318
Quinones QJ, de Ridder GG, Pizzo SV (2008) GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol 23(11):1409–1416
Blond-Elguindi S, Fourie AM, Sambrook JF, Gething MJ (1993) Peptide-dependent stimulation of the ATPase activity of the molecular chaperone BiP is the result of conversion of oligomers to active monomers. J Biol Chem 268(17):12730–12735
Ito D, Tanaka K, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y (2001) Up-regulation of the Ire1-mediated signaling molecule, Bip, in ischemic rat brain. Neuroreport 12(18):4023–4028
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666
Kraskiewicz H, FitzGerald U (2011) Partial XBP1 knockdown does not affect viability of oligodendrocyte precursor cells exposed to new models of hypoxia and ischemia in vitro. J Neurosci Res 89(5):661–673. doi:10.1002/jnr.22583
Barateiro A, Vaz AR, Silva SL, Fernandes A, Brites D (2012) ER stress, mitochondrial dysfunction and calpain/JNK activation are involved in oligodendrocyte precursor cell death by unconjugated bilirubin. Neruomol Med 14(4):285–302. doi:10.1007/s12017-012-8187-9
Ye Z, Connor JR (2000) Identification of iron responsive genes by screening cDNA libraries from suppression subtractive hybridization with antisense probes from three iron conditions. Nucleic Acids Res 28(8):1802–1807
Petrak J, Myslivcova D, Man P, Cmejla R, Cmejlova J, Vyoral D, Elleder M, Vulpe CD (2007) Proteomic analysis of hepatic iron overload in mice suggests dysregulation of urea cycle, impairment of fatty acid oxidation, and changes in the methylation cycle. Am J Physiol Gastrointest Liver Physiol 292(6):G1490–1498. doi:10.1152/ajpgi.00455.2006
Oliveira SJ, de Sousa M, Pinto JP (2011) ER stress and iron homeostasis: a new frontier for the UPR. Biochem Res Int 2011:896474. doi:10.1155/2011/896474
Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ (2008) Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 48(5):1644–1654. doi:10.1002/hep.22498
Li Q, Su D, O’Rourke B, Pogwizd SM, Zhou L (2014) Mitochondria-derived ROS bursts disturb calcium cycling and induce abnormal automaticity in guinea Pig cardiomyocyte: a theoretical study. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00493.2014
Son SM, Byun J, Roh SE, Kim SJ, Mook-Jung I (2014) Reduced IRE1alpha mediates apoptotic cell death by disrupting calcium homeostasis via the InsP3 receptor. Cell Death Dis 5, e1188. doi:10.1038/cddis.2014.129
Bhandary B, Marahatta A, Kim HR, Chae HJ (2012) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 14(1):434–456. doi:10.3390/ijms14010434
Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S et al (2012) Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct 2012:329635. doi:10.1155/2012/329635
Tan TC, Crawford DH, Jaskowski LA, Subramaniam VN, Clouston AD, Crane DI, Bridle KR, Anderson GJ et al (2013) Excess iron modulates endoplasmic reticulum stress-associated pathways in a mouse model of alcohol and high-fat diet-induced liver injury. Lab Investig 93(12):1295–1312. doi:10.1038/labinvest.2013.121
Lou LX, Geng B, Chen Y, Yu F, Zhao J, Tang CS (2009) Endoplasmic reticulum stress involved in heart and liver injury in iron-loaded rats. Clin Exp Pharmacol Physiol 36(7):612–618. doi:10.1111/j.1440-1681.2008.05114.x
Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M (2004) Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett 357(2):127–130. doi:10.1016/j.neulet.2003.12.080
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX et al (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182(2):367–377
Baud O, Li J, Zhang Y, Neve RL, Volpe JJ, Rosenberg PA (2004) Nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. Eur J Neurosci 20(7):1713–1726. doi:10.1111/j.1460-9568.2004.03616.x
Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D et al (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15(5):481–490. doi:10.1038/ncb2738
Oh SH, Lim SC (2009) Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther 329(1):112–122. doi:10.1124/jpet.108.144113
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4(12), e423. doi:10.1371/journal.pbio.0040423
Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M et al (2009) ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci 122(Pt 10):1626–1636. doi:10.1242/jcs.045625
Trousson A, Makoukji J, Petit PX, Bernard S, Slomianny C, Schumacher M, Massaad C (2009) Cross-talk between oxysterols and glucocorticoids: differential regulation of secreted phopholipase A2 and impact on oligodendrocyte death. PLoS ONE 4(11), e8080. doi:10.1371/journal.pone.0008080
Acknowledgments
This study was supported by research grant R-181-000-120-213 from the National Medical Research Council (NMRC) and R-181-000-148-750 from the National University Health System (NUHS), Singapore.
Conflict of Interest
The authors do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathnasamy, G., Murugan, M., Ling, EA. et al. Hypoxia-Induced Iron Accumulation in Oligodendrocytes Mediates Apoptosis by Eliciting Endoplasmic Reticulum Stress. Mol Neurobiol 53, 4713–4727 (2016). https://doi.org/10.1007/s12035-015-9389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9389-6