Abstract
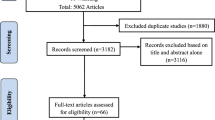
Mitochondrial dysfunction has been reported to be involved in the pathophysiology of autism spectrum disorder (ASD). Studies investigating the possible association between ASD and polymorphism in SLC25A12, which encodes the mitochondrial aspartate/glutamate carrier, have yielded inconsistent results. We conducted a systematic review and meta-analysis of such studies to elucidate if and which SLC25A12 single nucleotide polymorphisms (SNPs) are associated with ASD. We searched PubMed, Ovid, Web of Science, and ERIC databases through September 20th, 2014. Odds ratios (ORs) were aggregated using random effect models. Sensitivity analyses were conducted based on study design (family-based or case-control). Fifteen out of 79 non-duplicate records were retained for qualitative synthesis. We pooled 10 datasets from 9 studies with 2001 families, 735 individuals with ASD and 632 typically developing (TD) individuals for the meta-analysis of rs2292813, as well as 11 datasets from 10 studies with 2016 families, 852 individuals with ASD and 1058 TD individuals for the meta-analysis of rs2056202. We found a statistically significant association between ASD and variant in rs2292813 (OR = 1.190, 95 % CI 1.052–1.346, P = 0.006) as well as in rs2056202 (OR = 1.206, 95 % CI 1.035–1.405, P = 0.016). Sensitivity analyses including only studies with family-based design demonstrated significant association between ASD and polymorphism in rs2292813 (OR = 1.216, 95 % CI 1.075–1.376, P = 0.002) and rs2056202 (OR = 1.267, 95 % CI 1.041–1.542, P = 0.018). In contrast, sensitivity analyses including case-control design studies only failed to find a significant association. Further research on the role of SLC25A12 and ASD may pave the way for potential innovative therapeutic interventions.



Similar content being viewed by others
References
American Pychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington, VA, USA
Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T et al (2014) Oxytocin improves behavioural and neural deficits in inferring others' social emotions in autism. Brain 137(Pt 11):3073–3086
Centers for Disease Control and Prevention (2012) Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ 61(3):1–19
Frazier TW, Thompson L, Youngstrom EA, Law P, Hardan AY, Eng C, Morris N (2014) A twin study of heritable and shared environmental contributions to autism. J Autism Dev Disord 44(8):2013–2025
Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F, Plomin R, Ronald A (2011) Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5 %, 2.5 %, and 1 %). Arch Gen Psychiatry 68(11):1113–1121
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A et al (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11):1095–1102
Lai M-C, Lombardo MV, Baron-Cohen S (2014) Autism. Lancet 383(9920):896–910
Visscher PM, Brown MA, McCarthy MI, Yang J (2012) Five years of GWAS discovery. Am J Hum Genet 90(1):7–24
LoParo D, Waldman ID (2014) The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. doi:10.1038/mp.2014.77
Quiroz JA, Gray NA, Kato T, Manji HK (2008) Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33(11):2551–2565
Munakata K, Iwamoto K, Bundo M, Kato T (2005) Mitochondrial DNA 3243A > G mutation and increased expression of LARS2 gene in the brains of patients with bipolar disorder and schizophrenia. Biol Psychiatry 57(5):525–532
Rossignol DA, Frye RE (2012) Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry 17(3):290–314
Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G (2012) Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci 13(5):293–307
Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, Tassone F, Pessah IN (2010) Mitochondrial dysfunction in autism. JAMA 304(21):2389–2396
Kuwabara H, Yamasue H, Koike S, Inoue H, Kawakubo Y, Kuroda M, Takano Y, Iwashiro N et al (2013) Altered metabolites in the plasma of autism spectrum disorder: a capillary electrophoresis time-of-flight mass spectroscopy study. PLoS One 8(9):e73814
Aoki Y, Kasai K, Yamasue H (2012) Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry 2:e69
Aoki Y, Abe O, Yahata N, Kuwabara H, Natsubori T, Iwashiro N, Takano Y, Inoue H et al (2012) Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Transl Psychiatry 2:e178
Aoki Y, Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T et al (2014) Oxytocin's neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: a randomized controlled trial. Mol Psychiatry. doi:10.1038/mp.2014.74
Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS (2014) Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry 71(6):665–671
Picard M, Zhang J, Hancock S, Derbeneva O, Golhar R, Golik P, O'Hearn S, Levy S et al (2014) Progressive increase in mtDNA 3243A > G heteroplasmy causes abrupt transcriptional reprogramming. Proc Natl Acad Sci U S A 111(38):E4033–4042
Napolioni V, Persico AM, Porcelli V, Palmieri L (2011) The mitochondrial aspartate/glutamate carrier AGC1 and calcium homeostasis: physiological links and abnormalities in autism. Mol Neurobiol 44(1):83–92
Silverman JM, Buxbaum JD, Ramoz N, Schmeidler J, Reichenberg A, Hollander E, Angelo G, Smith CJ et al (2008) Autism-related routines and rituals associated with a mitochondrial aspartate/glutamate carrier SLC25A12 polymorphism. Am J Med Genet B Neuropsychiatr Genet 147(3):408–410
Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, Matsuzaki H, Miyachi T et al (2012) Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism 3(1):12
del Arco A, Satrustegui J (1998) Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J Biol Chem 273(36):23327–23334
Blasi F, Bacchelli E, Carone S, Toma C, Monaco AP, Bailey AJ, Maestrini E, International Molecular Genetic Study of Autism Consortium (IMGSAC) (2006) SLC25A12 and CMYA3 gene variants are not associated with autism in the IMGSAC multiplex family sample. Eur J Hum Genet 14(1):123–126
Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD (2004) Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry 161(4):662–669
Segurado R, Conroy J, Meally E, Fitzgerald M, Gill M, Gallagher L (2005) Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. Am J Psychiatry 162(11):2182–2184
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354(9193):1896–1900
Higgins J, Green S (2008) Cochrane handbook for systematic reviews of interventions. Wiley, Chichester, UK
Kazeem GR, Farrall M (2005) Integrating case-control and TDT studies. Ann Hum Genet 69(Pt3):329–335
Brandys MK, Kas MJ, van Elburg AA, Ophoff R, Slof-Op't Landt MC, Middeldorp CM, Boomsma DI, van Furth EF et al (2013) The Val66Met polymorphism of the BDNF gene in anorexia nervosa: new data and a meta-analysis. World J Biol Psychiatry 14(6):441–451
Kim SJ, Silva RM, Flores CG, Jacob S, Guter S, Valcante G, Zaytoun AM, Cook EH et al (2011) A quantitative association study of SLC25A12 and restricted repetitive behavior traits in autism spectrum disorders. Mol Autism 2(1):8
Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, Hager J, Rousseau F et al (2010) Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry 15(1):38–52
Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD (2008) An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet 147B(7):1152–1158
Carayol J, Schellenberg GD, Tores F, Hager J, Ziegler A, Dawson G (2010) Assessing the impact of a combined analysis of four common low-risk genetic variants on autism risk. Mol Autism 1:4
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Aoki Y, Aoki A, Suwa H (2012) Reduction of N-acetylaspartate in the medial prefrontal cortex correlated with symptom severity in obsessive-compulsive disorder: meta-analyses of 1H-MRS studies. Transl Psychiatry 2:1–10
Aoki Y, Inokuchi R, Suwa H, Aoki A (2013) Age-related change of neurochemical abnormality in attention-deficit hyperactivity disorder: a meta-analysis. Neurosci Biobehav Rev 37(8):1692–1701
Aoki Y, Abe O, Nippashi Y, Yamasue H (2013) Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism 4(1):25
Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H (2012) Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J Neurol Neurosurg Psychiatry 83(9):870–876
Chien WH, Wu YY, Gau SS, Huang YS, Soong WT, Chiu YN, Chen CH (2010) Association study of the SLC25A12 gene and autism in Han Chinese in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry 34(1):189–192
Correia C, Coutinho AM, Diogo L, Grazina M, Marques C, Miguel T, Ataíde A, Almeida J et al (2006) Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord 36(8):1137–1140
Durdiakova J, Warrier V, Baron-Cohen S, Chakrabarti B (2014) Single nucleotide polymorphism rs6716901 in SLC25A12 gene is associated with Asperger syndrome. Mol Autism 5(1):25
Lepagnol-Bestel AM, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide AL, Moalic JM, Muller D et al (2008) SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry 13(4):385–397
Prandini P, Pasquali A, Malerba G, Marostica A, Zusi C, Xumerle L, Muglia P, Da Ros L et al (2012) The association of rs4307059 and rs35678 markers with autism spectrum disorders is replicated in Italian families. Psychiatr Genet 22(4):177–181
Rabionet R, McCauley JL, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, Haines JL, DeLong GR et al (2006) Lack of association between autism and SLC25A12. Am J Psychiatry 163(5):929–931
Turunen JA, Rehnstrom K, Kilpinen H, Kuokkanen M, Kempas E, Ylisaukko-Oja T (2008) Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Res 1(3):189–192
Yan Z-H, Xing J, Luo H-Y, Yang T-S, Sakamoto Y, Nanba E (2006) Association between SLC25A12 and SCN2A2 gene polymorphisms and autism. J Jilin Univ Med Ed 32:313–315
Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M et al (2009) Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459(7246):528–533
Weiss LA, Arking DE, Gene Discovery Project of Johns H, the Autism C, Daly MJ, Chakravarti A (2009) A genome-wide linkage and association scan reveals novel loci for autism. Nature 461(7265):802–808
Wittkowski KM, Sonakya V, Bigio B, Tonn MK, Shic F, Ascano M, Nasca C, Gold-Von SG (2014) A novel computational biostatistics approach implies impaired dephosphorylation of growth factor receptors as associated with severity of autism. Transl Psychiatry 4:e354
Baslow MH (2011) The vertebrate brain, evidence of its modular organization and operating system: insights into the brain’s basic units of structure, function, and operation and how they influence neuronal signaling and behavior. Front Behav Neurosci 5:5
Sager T, Hansen A, Laursen H (2000) Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab 20(5):780–788
Moffett JR, ROSS B, Arun P, Madhavarao CN, Namboodiri AMA (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81(2):89–131
Kamada K, Takeuchi F, Houkin K, Kitagawa M, Kuriki S, Ogata A, Tashiro K, Koyanagi I et al (2001) Reversible brain dysfunction in MELAS: MEG, and (1)H MRS analysis. J Neurol Neurosurg Psychiatry 70(5):675–678
Satrústegui J, Contreras L, Ramos M, Marmol P, del Arco A, Saheki T, Pardo B (2007) Role of aralar, the mitochondrial transporter of aspartate-glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res 85(15):3359–3366
Jalil MA, Begum L, Contreras L, Pardo B, Iijima M, Li MX, Ramos M, Marmol P et al (2005) Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem 280(35):31333–31339
Ramos M, Pardo B, Llorente-Folch I, Saheki T, Del Arco A, Satrustegui J (2011) Deficiency of the mitochondrial transporter of aspartate/glutamate aralar/AGC1 causes hypomyelination and neuronal defects unrelated to myelin deficits in mouse brain. J Neurosci Res 89(12):2008–2017
Falk MJ, Li D, Gai X, McCormick E, Place E, Lasorsa FM, Otieno FG, Hou C et al (2014) AGC1 deficiency causes infantile epilepsy, abnormal myelination, and reduced N-acetylaspartate. JIMD Rep 14:77–85
Tebartz van Elst L, Maier S, Fangmeier T, Endres D, Mueller GT, Nickel K, Ebert D, Lange T et al (2014) Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: evidence in support of the excitatory/inhibitory imbalance hypothesis. Mol Psychiatry. doi:10.1038/mp.2014.62
Horder J, Lavender T, Mendez MA, O'Gorman R, Daly E, Craig MC, Lythgoe DJ, Barker GJ et al (2013) Reduced subcortical glutamate/glutamine in adults with autism spectrum disorders: a [(1)H]MRS study. Transl Psychiatry 3:e279
McGinnis R, Shifman S, Darvasi A (2002) Power and efficiency of the TDT and case-control design for association scans. Behav Genet 32(2):135–144
Author contributions
YA conceived the study design. YA and SC have independently screened and extracted the data. YA and SC wrote the paper.
Conflict of Interest
Dr. Samuele Cortese has received royalties from Aargon Healthcare Italy.
Dr. Yuta Aoki declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Aoki, Y., Cortese, S. Mitochondrial Aspartate/Glutamate Carrier SLC25A12 and Autism Spectrum Disorder: a Meta-Analysis. Mol Neurobiol 53, 1579–1588 (2016). https://doi.org/10.1007/s12035-015-9116-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9116-3