Abstract
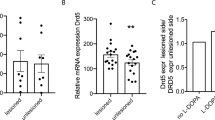
Levodopa-induced dyskinesias (LID) are a frequent complication of Parkinson’s disease pharmacotherapy that causes significant disability and narrows the therapeutic window. Pharmacological management of LID is challenging partly because the precise molecular mechanisms are not completely understood. Here, our aim was to determine molecular changes that could unveil targetable mechanisms underlying this drug complication. We examined the expression and downstream activity of dopamine receptors (DR) in the striatum of 1-methyl-4-phenyl-1,2,3,6 tetrahydropiridine (MPTP)-lesioned monkeys with and without l-DOPA treatment. Four monkeys were made dyskinetic and other four received a shorter course of l-DOPA and did not develop LID. Our results show that l-DOPA treatment induces an increase in DRD2 and DRD3 expression in the postcommissural putamen, but only DRD3 is correlated with the severity of LID. Dyskinetic monkeys show a hyperactivation of the canonical DRD1-signaling pathway, measured by an increased phosphorylation of protein kinase A (PKA) and its substrates, particularly DARPP32. In contrast, activation of the DRD2-signaling pathway, visible in the levels of Akt phosphorylated on Thr308 and GSK3β on Ser9, is associated with l-DOPA treatment, independently of the presence of dyskinesias. Our data clearly demonstrate that dyskinetic monkeys present a dysregulation of the DRD3 receptor and the DRD1 pathway with a sustained increase of PKA activity in the postcommissural putamen. Importantly, we found that all signaling changes related to long-term l-DOPA administration are exquisitely restricted to the postcommissural putamen, which may be related to the recurrent failure of pharmacological approaches.




Similar content being viewed by others
References
Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA (1992) Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov Disord 7:117–124
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM (2013) The pharmacology of l-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 65:171–222
Pechevis M, Clarke CE, Vieregge P, Khoshnood B, Deschaseaux-Voinet C, Berdeaux G, Ziegler M (2005) Effects of dyskinesias in Parkinson’s disease on quality of life and health-related costs: a prospective European study. Eur J Neurol 12:956–963
Hung SW, Adeli GM, Arenovich T, Fox SH, Lang AE (2010) Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1112–1115
Bargiotas P, Konitsiotis S (2013) Levodopa-induced dyskinesias in Parkinson’s disease: emerging treatments. Neuropsychiatr Dis Treat 9:1605–1617
Murer MG, Moratalla R (2011) Striatal signaling in l-DOPA-induced dyskinesia: common mechanisms with drug abuse and long term memory involving D1 dopamine receptor stimulation. Front Neuroanat 5:51
Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci 6:501–506
Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G (2007) Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in l-DOPA-induced dyskinesia. J Neurosci 27:6995–7005
Aristieta A, Azkona G, Sagarduy A, Miguelez C, Ruiz-Ortega JA, Sanchez-Pernaute R, Ugedo L (2012) The role of the subthalamic nucleus in l-DOPA induced dyskinesia in 6-hydroxydopamine lesioned rats. PLoS One 7:e42652
Azkona G, Sagarduy A, Aristieta A, Vazquez N, Zubillaga V, Ruiz-Ortega JA, Perez-Navarro E, Ugedo L, Sanchez-Pernaute R (2014) Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in l-DOPA-treated rats. Neuropharmacology 79:726–737
Gerfen CR, Paletzki R, Worley P (2008) Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal-regulated kinase. J Neurosci 28:7113–7120
Santini E, Sgambato-Faure V, Li Q, Savasta M, Dovero S, Fisone G, Bezard E (2010) Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in l-DOPA-induced dyskinesia. PLoS One 5:e12322
Martinez A, Macheda T, Morgese MG, Trabace L, Giuffrida A (2012) The cannabinoid agonist WIN55212-2 decreases l-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res 72:236–242
Oh JD, Del Dotto P, Chase TN (1997) Protein kinase A inhibitor attenuates levodopa-induced motor response alterations in the hemi-parkinsonian rat. Neurosci Lett 228:5–8
Lebel M, Chagniel L, Bureau G, Cyr M (2010) Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiol Dis 38:59–67
Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG (2007) Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci 27:881–885
Bychkov E, Ahmed MR, Dalby KN, Gurevich EV (2007) Dopamine depletion and subsequent treatment with l-DOPA, but not the long-lived dopamine agonist pergolide, enhances activity of the Akt pathway in the rat striatum. J Neurochem 102:699–711
Morissette M, Samadi P, Hadj Tahar A, Belanger N, Di Paolo T (2010) Striatal Akt/GSK3 signaling pathway in the development of l-Dopa-induced dyskinesias in MPTP monkeys. Prog Neuropsychopharmacol Biol Psychiatry 34:446–454
Morissette M, Goulet M, Grondin R, Blanchet P, Bedard PJ, Di Paolo T, Levesque D (1998) Associative and limbic regions of monkey striatum express high levels of dopamine D3 receptors: effects of MPTP and dopamine agonist replacement therapies. Eur J Neurosci 10:2565–2573
Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC (1997) Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A 94:3363–3367
Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P (2003) Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med 9:762–767
Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R (2008) Identification of dopamine D1–D3 receptor heteromers: indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem 283:26016–26025
Bezard E, Przedborski S (2011) A tale on animal models of Parkinson’s disease. Mov Disord 26:993–1002
San Sebastian W, Guillen J, Manrique M, Belzunegui S, Ciordia E, Izal-Azcarate A, Garrido-Gil P, Vazquez-Claverie M, Luquin MR (2007) Modification of the number and phenotype of striatal dopaminergic cells by carotid body graft. Brain 130:1306–1316
Vazquez-Claverie M, Garrido-Gil P, San Sebastian W, Izal-Azcarate A, Belzunegui S, Marcilla I, Lopez B, Luquin MR (2009) Acute and chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administrations elicit similar microglial activation in the substantia nigra of monkeys. J Neuropathol Exp Neurol 68:977–984
Luquin MR, Montoro RJ, Guillen J, Saldise L, Insausti R, Del Rio J, Lopez-Barneo J (1999) Recovery of chronic parkinsonian monkeys by autotransplants of carotid body cell aggregates into putamen. Neuron 22:743–750
Sanchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O (2007) In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiol Dis 27:220–227
Azkona G, Levannon D, Groner Y, Dierssen M (2010) In vivo effects of APP are not exacerbated by BACE2 co-overexpression: behavioural characterization of a double transgenic mouse model. Amino Acids 39:1571–1580
Xiang L, Szebeni K, Szebeni A, Klimek V, Stockmeier CA, Karolewicz B, Kalbfleisch J, Ordway GA (2008) Dopamine receptor gene expression in human amygdaloid nuclei: elevated D4 receptor mRNA in major depression. Brain Res 1207:214–224
Potts LF, Wu H, Singh A, Marcilla I, Luquin MR, Papa SM (2013) Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp Neurol. doi:10.1016/j.expneurol.2013.09.014
Ordonez C, Moreno-Murciano P, Hernandez M, Di Caudo C, Carril-Mundinano I, Vazquez N, Sanchez-Pernaute R, Luquin MR (2013) Sox-2 positive neural progenitors in the primate striatum undergo dynamic changes after dopamine denervation. PLoS One 8(6):e66377
Cheng X, Ma Y, Moore M, Hemmings BA, Taylor SS (1998) Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc Natl Acad Sci U S A 95:9849–9854
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151
Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P (1992) Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A 89:8155–8159
Richtand NM, Welge JA, Levant B, Logue AD, Hayes S, Pritchard LM, Geracioti TD, Coolen LM, Berger SP (2003) Altered behavioral response to dopamine D3 receptor agonists 7-OH-DPAT and PD 128907 following repetitive amphetamine administration. Neuropsychopharmacology 28:1422–1432
van Kampen JM, Stoessl AJ (2003) Effects of oligonucleotide antisense to dopamine D3 receptor mRNA in a rodent model of behavioural sensitization to levodopa. Neuroscience 116:307–314
Blanchet PJ, Konitsiotis S, Chase TN (1997) Motor response to a dopamine D3 receptor preferring agonist compared to apomorphine in levodopa-primed 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. J Pharmacol Exp Ther 283:794–799
Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR (2009) Evaluation of the D3 dopamine receptor selective antagonist PG01037 on l-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology 56:944–955
Kumar R, Riddle LR, Griffin SA, Chu W, Vangveravong S, Neisewander J, Mach RH, Luedtke RR (2009) Evaluation of D2 and D3 dopamine receptor selective compounds on l-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology 56:956–969
Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B (2009) Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of l-DOPA-induced dyskinesia. J Neurosci 29:4829–4835
Bagetta V, Sgobio C, Pendolino V, Del Papa G, Tozzi A, Ghiglieri V, Giampa C, Zianni E, Gardoni F, Calabresi P, Picconi B (2012) Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson’s disease. J Neurosci 32:17921–17931
Pavon N, Martin AB, Mendialdua A, Moratalla R (2006) ERK phosphorylation and FosB expression are associated with l-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry 59:64–74
Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA (2007) Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of l-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry 62:800–810
Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R (2009) Genetic inactivation of dopamine D1 but not D2 receptors inhibits l-DOPA-induced dyskinesia and histone activation. Biol Psychiatry 66:603–613
Lindenbach D, Dupre KB, Eskow Jaunarajs KL, Ostock CY, Goldenberg AA, Bishop C (2013) Effects of 5-HT1A receptor stimulation on striatal and cortical M1 pERK induction by l-DOPA and a D1 receptor agonist in a rat model of Parkinson’s disease. Brain Res 1537:327–339
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123(Pt 11):2297–2305
Beaulieu JM, Gainetdinov RR, Caron MG (2007) The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci 28:166–172
Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR (2011) Beyond cAMP: the regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci 4:38
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20:91–127
Bourdenx M, Nilsson A, Wadensten H, Falth M, Li Q, Crossman AR, Andren PE, Bezard E (2013) Abnormal structure-specific peptide transmission and processing in a primate model of Parkinson’s disease and l-DOPA-induced dyskinesia. Neurobiol Dis 62C:307–312
Acknowledgments
We thank Dr. Lanciego for providing control monkey brains. This study was supported by grants from the Department of Industry of the Basque Government, S-PE12UN030 (RSP) and from the Spanish Health Ministry (FIS PI08/1866 to MRL and FIS PI13/01250 to EP-N).
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Dopamine receptor protein expression in the pre-commissural striatum. Western blot representative images (upper panel) and quantification (lower panel) of (a) DRD1, (b) DRD2 and (c) DRD3 expression in the caudate (left) and the putamen (right). Groups: Control (C; n = 3), MPTP-lesioned saline (P; n = 3), Non-dyskinetic (ND; n = 4) and Dyskinetic (D; n = 4). Data represent median and ranges. (GIF 299 kb)
Supplementary Figure 2
Phosphorylation of PKA and its substrates in the postcommissural caudate. Western blot representative images (upper panel) and quantification (lower panel). (a) pSer(Thr) PKA substrates, (b) pPKAc, (c) pDARPP32, (d) pSTEP61 and (d) pERK2 levels. Groups: Control (C; n = 3), MPTP-lesioned saline (P; n = 3), Non-dyskinetic (ND; n = 4) and Dyskinetic (D; n = 4). Data represent median and ranges. (GIF 134 kb)
Supplementary Figure 3
DRD2 signaling pathway in the postcommissural caudate. Western blot representative images (upper panel) and quantification (lower panel). (a) pAkt Thr308, (b) pGSK3 Tyr216 and, (c) pGSK3 Ser9 levels. Groups: Control (C; n = 3), MPTP-lesioned saline (P; n = 3), Non-dyskinetic (ND; n = 4) and Dyskinetic (D; n = 4). Data represent median and ranges. (GIF 169 kb)
Table S1
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
Azkona, G., Marcilla, I., López de Maturana, R. et al. Sustained Increase of PKA Activity in the Postcommissural Putamen of Dyskinetic Monkeys. Mol Neurobiol 50, 1131–1141 (2014). https://doi.org/10.1007/s12035-014-8688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8688-7