Abstract
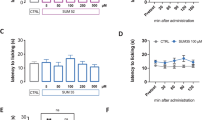
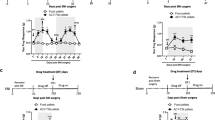
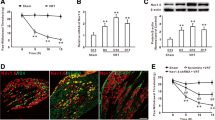
Recent studies have shown that histone deacetylase (HDAC) inhibitors can alleviate inflammatory and neuropathic pain. We investigated the effects of JNJ-26481585, a pan-HDAC inhibitor on basal mechanical sensitivity. Unlike previous reports for HDAC inhibitors, JNJ-26481585 induced mechanical hypersensitivity in mice. This effect was reversible with gabapentin. Voltage-dependent calcium channel subunit alpha-2/delta-1, one of the putative targets for gabapentin, was upregulated in the spinal cord from JNJ-26481585-treated mice. Transcriptional profiling of spinal cord from JNJ-26481585-treated mice showed significant alterations in pathways involved in axon guidance, suggesting overlap in mechanisms underlying neurotoxicity caused by other known chemotherapeutic agents. To investigate the mechanisms underlying the development of pain, RAW 264.7 mouse macrophage cells were treated with JNJ-26481585. There was a dose- and time-dependent activation of nuclear factor-kappaB and interleukin-1β increase. Thus, alterations in the axon guidance pathway, increase in voltage-dependent calcium channel alpha(2)delta-1 subunit, and the induction of proinflammatory mediators by JNJ-26481585 could all contribute to increased mechanical sensitivity. Our data indicate that the effect of HDAC inhibitors may be unique to the compound studied and highlights the potential to develop chemotherapy-induced peripheral neuropathy with the use of a pan-HDAC inhibitor for cancer treatment, and this pain may be alleviated by gabapentin.





Similar content being viewed by others
References
Akimova T, Beier UH, Liu Y, Wang L, Hancock WW (2012) Histone/protein deacetylases and T-cell immune responses. Blood 119:2443–2451
Arts J, King P, Marien A, Floren W, Belien A, Janssen L, Pilatte I, Roux B, Decrane L, Gilissen R, Hickson I, Vreys V, Cox E, Bol K, Talloen W, Goris I, Andries L, Du Jardin M, Janicot M, Page M, van Emelen K, Angibaud P (2009) JNJ-26481585, a novel "second-generation" oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res 15:6841–6851
Austin PJ, Moalem-Taylor G (2010) The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 229:26–50
Bai G, Wei D, Zou S, Ren K, Dubner R (2010) Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain 6:51
Bode KA, Schroder K, Hume DA, Ravasi T, Heeg K, Sweet MJ, Dalpke AH (2007) Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology 122:596–606
Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, Huang Q (2007) Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood 109:1123–1130
Cavaletti G, Marmiroli P (2010) Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol 6:657–666
Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RWT, Copani A, Nicoletti F (2009) Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol 75:1014–1020
Christo PJ, Mazloomdoost D (2008) Cancer pain and analgesia. Ann N Y Acad Sci 1138:278–298
Denk F, Huang W, Sidders B, Bithell A, Crow M, Grist J, Sharma S, Ziemek D, Rice ASC, Buckley NJ, McMahon SB (2013) HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain 154:1668–1679
Denk F, McMahon SB (2012) Chronic pain: emerging evidence for the involvement of epigenetics. Neuron 73:435–444
Doehring A, Geisslinger G, Lotsch J (2011) Epigenetics in pain and analgesia: an imminent research field. Eur J Pain 15:11–16
Gauchan P, Andoh T, Ikeda K, Fujita M, Sasaki A, Kato A, Kuraishi Y (2009) Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: different effectiveness of gabapentin and different expression of voltage-dependent calcium channel alpha(2)delta-1 subunit. Biol Pharm Bull 32:732–734
Geranton SM (2012) Targeting epigenetic mechanisms for pain relief. Curr Opin Pharmacol 12:35–41
Giger RJ, Hollis ER 2nd, Tuszynski MH (2010) Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2:a001867
Grond S, Radbruch L, Meuser T, Sabatowski R, Loick G, Lehmann KA (1999) Assessment and treatment of neuropathic cancer pain following WHO guidelines. Pain 79:15–20
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10:32–42
Halili MA, Andrews MR, Labzin LI, Schroder K, Matthias G, Cao C, Lovelace E, Reid RC, Le GT, Hume DA, Irvine KM, Matthias P, Fairlie DP, Sweet MJ (2010) Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. J Leukoc Biol 87:1103–1114
Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, Eilbeck K, Lewis S, Marshall B, Mungall C, Richter J, Rubin GM, Blake JA, Bult C, Dolan M, Drabkin H, Eppig JT, Hill DP, Ni L, Ringwald M, Balakrishnan R, Cherry JM, Christie KR, Costanzo MC, Dwight SS, Engel S, Fisk DG, Hirschman JE, Hong EL, Nash RS, Sethuraman A, Theesfeld CL, Botstein D, Dolinski K, Feierbach B, Berardini T, Mundodi S, Rhee SY, Apweiler R, Barrell D, Camon E, Dimmer E, Lee V, Chisholm R, Gaudet P, Kibbe W, Kishore R, Schwarz EM, Sternberg P, Gwinn M, Hannick L, Wortman J, Berriman M, Wood V, de la Cruz N, Tonellato P, Jaiswal P, Seigfried T, White R (2004) The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res 32:D258–D261
Hu H-J, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau Iv RW (2006) The Kv4.2 potassium channel subunit is required for pain plasticity. Neuron 50:89–100
Huang DW, Sherman BT, Lempicki RA (2008) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 4:44–57
Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR (2011) Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63:772–810
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Jaggi AS, Singh N (2012) Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 291:1–9
Kaley TJ, Deangelis LM (2009) Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol 145:3–14
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Kiguchi N, Kobayashi Y, Maeda T, Fukazawa Y, Tohya K, Kimura M, Kishioka S (2012) Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J Pharmacol Exp Ther 340:577–587
Klein R (2009) Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci 12:15–20
Kouzarides T (2007) SnapShot: histone-modifying enzymes. Cell 131:822
Levy MH, Chwistek M, Mehta RS (2008) Management of chronic pain in cancer survivors. Cancer J 14:401–409
Liang D-Y, Li X, Clark JD (2013) Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 14:36–47
Malik B, Stillman M (2008) Chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep 8:56–65
Matsumoto M, Inoue M, Hald A, Xie W, Ueda H (2006) Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther 318:735–740
Mehlen P, Delloye-Bourgeois C, Chedotal A (2011) Novel roles for slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer 11:188–197
Pachman DR, Barton DL, Watson JC, Loprinzi CL (2011) Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 90:377–387
Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC (2008) Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem 15:3081–3094
Pasterkamp RJ, Giger RJ (2009) Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol 19:263–274
Polomano RC, Bennett GJ (2001) Chemotherapy-evoked painful peripheral neuropathy. Pain Med 2:8–14
Roger T, Lugrin J, Le Roy D, Goy G, Mombelli M, Koessler T, Ding XC, Chanson A-L, Reymond MK, Miconnet I, Schrenzel J, François P, Calandra T (2011) Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood 117:1205–1217
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ (2011) Histone deacetylases as regulators of inflammation and immunity. Trends Immunol 32:335–343
Sweet MJ, Shakespear MR, Kamal NA, Fairlie DP (2012) HDAC inhibitors: modulating leukocyte differentiation, survival, proliferation and inflammation. Immunol Cell Biol 90:14–22
Tong WG, Wei Y, Stevenson W, Kuang SQ, Fang Z, Zhang M, Arts J, Garcia-Manero G (2010) Preclinical antileukemia activity of JNJ-26481585, a potent second-generation histone deacetylase inhibitor. Leuk Res 34:221–228
Tran L, Chaloner A, Sawalha AH, Greenwood Van-Meerveld B (2013) Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 38:898–906
Venugopal B, Baird R, Kristeleit R, Plummer R, Cowan R, Stewart A, Fourneau N, Hellemans P, Elsayed Y, McClue S, Smit JW, Forslund A, Phelps C, Camm J, Evans TRJ, de Bono JS, Banerji U (2013) A phase I study of quisinostat (JNJ-26481585), an oral hydroxamate histone deacetylase inhibitor, in patients with advanced solid tumors. Clin Cancer Res
Ververis K, Karagiannis TC (2011) Potential non-oncological applications of histone deacetylase inhibitors. Am J Trans Res 3:454–467
Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C (2008) Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 44:1507–1515
Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 107:135–142
Zhang Z, Cai Y-Q, Zou F, Bie B, Pan ZZ (2011) Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 17:1448–1455
Acknowledgments
This study was supported by funds from Drexel University College of Medicine and Rita Allen Foundation grant to Seena Ajit. We thank members of the Kimmel Cancer Center Cancer for microarray processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kathryn E. Capasso and Melissa T. Manners contributed equally to this work.
Rights and permissions
About this article
Cite this article
Capasso, K.E., Manners, M.T., Quershi, R.A. et al. Effect of Histone Deacetylase Inhibitor JNJ-26481585 in Pain. J Mol Neurosci 55, 570–578 (2015). https://doi.org/10.1007/s12031-014-0391-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0391-7