Abstract
Background
Several classes of histone deacetylases (HDACs) are expressed in the spinal cord that is a critical structure of the nociceptive pathway. HDAC-regulated histone acetylation is an important component of chromatin remodeling leading to epigenetic regulation of gene transcription. To understand the role of histone acetylation in epigenetic regulation of pathological pain, we have studied the impact of different classes of HDACs in the spinal cord on inflammatory hyperalgesia induced by complete Freund's adjuvant (CFA).
Results
We intrathecally applied inhibitors specific to different classes of HDACs and evaluated their impact on inflammatory hyperalgesia. Pre-injected inhibitors targeting class I as well as II (SAHA, TSA, LAQ824) or IIa (VPA, 4-PB) HDACs significantly delayed the thermal hyperalgesia induced by unilateral CFA injection in the hindpaw. Existing hyperalgesia induced by CFA was also attenuated by the HDAC inhibitors (HDACIs). In contrast, these inhibitors did not interfere with the thermal response either in naïve animals, or on the contralateral side of inflamed animals. Interestingly, MS-275 that specifically inhibits class I HDACs failed to alter the hyperalgesia although it increased histone 3 acetylation in the spinal cord as SAHA did. Using immunoblot analysis, we further found that the levels of class IIa HDAC members (HDAC4, 5, 7, 9) in the spinal dorsal horn were upregulated following CFA injection while those of class I HDAC members (HDAC1, 2, 3) remained stable or were slightly reduced.
Conclusions
Our data suggest that activity of class II HDACs in the spinal cord is critical to the induction and maintenance of inflammatory hyperalgesia induced by CFA, while activity of class I HDACs may be unnecessary. Comparison of the effects of HDACIs specific to class II and IIa as well as the expression pattern of different HDACs in the spinal cord in response to CFA suggests that the members of class IIa HDACs may be potential targets for attenuating persistent inflammatory pain.
Similar content being viewed by others
Background
Gene expression in the nociceptive pathway plays an important role in the induction and maintenance of persistent pain, including inflammatory pain resulting from tissue damage [1–3]. It has been found that dynamic changes in the steady-state levels of mRNAs and/or proteins in the peripheral and central nervous system occur during the development of pathological pain and that animals with specific gene knockout or knockdown exhibit altered nociceptive responses and different sensitivity to the development of pathological pain [1, 4]. However, the molecular mechanisms underlying the changes of mRNA and protein levels in pathological pain conditions mostly remain unexplored except that the status of a few transcription factors, e.g., deletion of DREAM [5] and modifications of CREB [6] and NF-κB [7], were studied as single transcription factors and as a result of activated signal pathways, and mutations in a few genes have been found to be associated with the alteration of pain sensitivity in humans [8].
In addition to genetic mechanisms, gene transcription in eukaryotes is recently known to be subject to epigenetic regulation that is independent of genomic DNA sequences and is influenced largely by environmental and developmental factors [9, 10]. Chromatin remodeling, DNA methylation and noncoding RNAs are three known mechanisms of epigenetic regulation [10–12]. The major force in chromatin remodeling is the modification of histone N-terminal tails [13]. One of these modifications is the acetylation of the ε-amino group of conserved lysine residues [14, 15] that regulate transcription and facilitate neuronal plasticity, thus involving several neurological events [10, 13, 16–19]. Histone acetylation is catalyzed by histone acetyltransferase and removed by histone deacetylases (HDACs) [14, 20]. The mammalian genome contains at least 18 HDAC genes that express proteins grouped into four classes: class I (HDAC1, 2, 3, and 8), class II (HDAC4, 5, 7, 9 in IIa, and HDAC6, 10 in IIb), class III (sirtuin1~7) and class VI (HDAC11) [14, 20, 21]. These HDAC genes are differentially expressed in the nervous system [22–24]. For example, the spinal cord expresses the genes of HDAC1~8, and 11 [25–27]. Despite the finding that no mRNA of the HDAC9 and 10 genes was detected by in situ hybridization from the spinal cord [24], microarray data deposited to the UCSC database http://www.ucsc.edu and in situ hybridization data provided on-line by Allen Institute http://www.brain-map.org showed the presence of these mRNAs and those from all seven sirtuin genes in the spinal cord. However, the roles of different classes of HDACs in pain signal transmission in the spinal cord have not been explored.
Animal studies demonstrated that the nociceptive threshold increased in adult animals who experienced stress in pre- and post-natal periods [28–31], during which the nervous system is most sensitive to environmental changes and subjected to epigenetic regulation [32]. Human studies indicated that the genetic impact on pain sensitivity in monozygotic twins diminished with increasing age that apparently accompanies more environmental exposures [33]. These observations suggest that nociceptive sensitivity may be modified by environmental and developmental factors in a way independent of genetic mechanisms. To support this notion, it was found that valproic acid (VPA) used as an antiepileptic drug for prophylactic treatment of migraine [34–37] and as an anticonvulsant to treat chronic cancer pain [38] may broadly inhibit HDACs [39–42], although VPA's effects on GABAergic activity, excitatory transmission and monoamines may affect nociception [43]. It was also found that mice expressing partial loss-of-function of HDAC4 exhibited reduced thermal nociception, but did not show a different response during the formalin test in comparison to wild-type littermates [44]. In a recent report, SAHA and MS-275 were used as HDAC inhibitors (HDACIs) after a consecutive 5-day systemic treatment and significantly reduced the second phase of the formalin test in mice [27]. Another recent report revealed that in a neuropathic pain model the neuron-restrictive silencer factor exhibits long-lasting upregulation in the dorsal root ganglion due to recruitment of histone 4 to the second promoter of the gene. Upregulated neuron-restrictive silencer factor may then suppress expression of the μ-opioid receptor and Nav1.8 genes in C-fibers [45]. Taken together, these studies suggest that epigenetic mechanisms may be involved in modification of nociception and pathological pain. However, it remains largely unknown whether the nociceptive pathway or which part of this pathway is involved. In addition, except for a differential subcellular distribution among HDACs [23, 46], the potential roles of each class HDAC in the development of pathological pain are still unknown.
In the present studies, we applied HDACIs, selective to different classes of HDACs, to the spinal cord and studied modification of the inflammatory thermal hyperalgesia induced by CFA in mice. We observed that the inhibition of class II HDACs is critical to attenuate inflammatory hyperalgesia and the expression of the members in class IIa HDACs in the spinal dorsal horn was upregulated at the protein level following CFA injection. In contrast, the inhibition of class I HDAC with MS-275 showed no effect on CFA-induced thermal hyperalgesia and in addition the expression of this class of HDACs in the spinal cord was not induced by CFA.
Results
CFA-injected mice exhibited significant peak hypersensitivity to a noxious heat stimulus at rest 30 min after the injection (P < 0.01 compared to the baseline before the injection, Fig. 1). This typical thermal hypersensitivity appeared only on the hindpaw ipsilateral to the injection side as reported [47]. The thermal hypersensitivity was slowly resolved by 14 days after the injection. In contrast, the contralateral hindpaw showed no significant changes compared to the baseline during the tested period (P > 0.05 compared to the baseline).
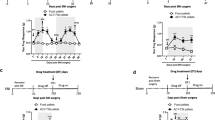
To explore the roles of HDACs in hyperalgesia, we applied different HDACIs to the spinal cord via intrathecal injection (i.t.). Considering that VPA was previously reported to reduce tactile allodynia in a neuropathic pain animal model after systemic administration [48] and is widely used as an HDACI to suppress class I and IIa HDACs [46, 49], we first tested this inhibitor. As shown in Fig. 2A, animals receiving vehicle injection exhibited peak thermal hyperalgesia in response to CFA after 30 min as those without i.t. shown in Fig. 1. This CFA-induced hypersensitivity was largely blocked in the early time period after VPA preinjection and even showed no significant difference from the baseline. This inhibition exhibited dose dependency as shown in the inserted bar graph at the one hour time point in Fig. 2A, and declined 5 hr after CFA injection, possibly due to the clearance of the one-time injected inhibitor from tissues. However, VPA is also known to interfere with GABAergic activity, excitatory transmission and monoamines that are involved in the development of pathological pain [43]. To confirm whether HDAC activity is really involved in the inhibition of hyperalgesia, we then tested three groups of HDACIs specific to different classes of HDACs. These inhibitors are SAHA, trichostatin A (TSA), and LAQ824 to target class I and II HDACs, 4-phenylbutyrate (4-PB) to inhibit class I and IIa HDACs as well as MS-275 to block class I HDACs only [19, 46, 50–54]. As shown in Fig. 2, CFA-induced thermal hyperalgesia was significantly attenuated by all HDACIs, except for MS-275, in comparison to vehicle. No significant alteration of CFA-induced effect was observed for MS-275, even though a maximally soluble dose of 0.5 μg MS-275 in a 5 μl injection volume has been used. The attenuation lasted for a short period of time approximately 3 hr or less after CFA injection and showed dose dependency as indicated in the inserted bar graphs for tested dose per injection of 1~25 μg for SAHA, 0.04~1 μg for TSA, 8~200 ng for LAQ824, and 10~250 μg for 4-PB. Of these tested HDACIs, LAQ824 showed similar inhibitory effects between 40 and 200 ng suggesting saturation above 40 ng dosage. In comparison, all other HDACIs showed increasing effect following doses used. This observation prompted us to evaluate the potential of each HDACI tested by comparing their inhibitory effects on hyperalgesia at the maximal doses 30 min after CFA (Fig. 2G). With the exception of VPA, SAHA produced the strongest inhibition of hyperalgesia among tested inhibitors specific to HDAC. In another group of studies, SAHA and VPA did not interfere with thermal nociception in naïve mice within the same tested time period as shown in Fig. 3.
Short-term attenuation effects of pre-injected HDACI on thermal hyperalgesia in mice. Mice received intrathecally injected vehicle or HDACI at indicated dose 30 min before unilateral injection of CFA. Paw withdrawal latency was measured before injections as baseline, and after CFA injections as hyperalgesia response. Following HDACIs were injected for panel (in parenthesis) VPA (A), 4-BP (B), SAHA (C), TSA (D), LAQ824 (E). The inserted bar graphs showed dose dependence from the time lined up with a dash line. Mean values of eight animals per group plus standard errors are presented for the responses on the ipsilateral side. * P < 0.01, # P < 0.05: compared to vehicle at the same time point. G. Comparison of inhibition of thermal hyperalgesia by tested HDACI. Inhibition of hyperalgesia by all inhibitors at the maximal dose tested 30 min after CFA injection were calculated as described in method. One-way ANOVA analysis indicated there was no significant difference among HDAC-specific inhibitors, but all of them showed significant difference in comparison to VPA (P < 0.05).
Effects of HDACI on thermal nociception on naïve mice. Naïve mice treated with i.t. SAHA (25 μg) or VPA (0.5 mg) were tested for their hindpaw response to a noxious heat beam for indicated time. Mean values + s.e. of PWL from 8 animals each group were presented. No significance was revealed for comparison among tested period either at the ipsilateral or contralateral side.
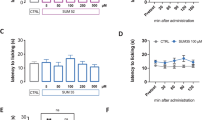
We then questioned whether injected MS-275 under the condition tested executed a similar pharmacological effect as other HDACIs did. The direct targets of these inhibitors are HDACs and the functional output of inhibition of these enzymes can be assessed by the examination of the histone acetylation. Given the fact that all HDACIs tested above retain the capability to pass the blood-brain barrier [20, 55–57], these inhibitors delivered intrathecally may primarily target the spinal cord and primary afferents. Therefore, we examined the effects of i.t. SAHA and MS-275 on histone 3 (H3) acetylation in the spinal cord in naïve mice. By immunoblot analysis, H3 acetylation was measured relative to total (pan-) H3 protein by an antibody specific to acetylated H3 lysine 9 residue (H3K9ac) and one to pan-H3, respectively. As shown in Fig. 4A and 4B, the relative H3K9ac signals in animals injected either with SAHA or with MS-275 were largely enhanced in comparison to that in animals receiving i.t. saline. Using an antibody specific to acetylated H3 lysine 9/18 (H3K9/18ac) for immunohistochemistry, we further observed that 30 min after the injection, the signals of H3K9/18ac robustly increased in the lumbar spinal cord (Fig. 4C). It is of interest to note that the superficial dorsal horn contained more H3K9/18ac signals. As revealed by double-labeling with NeuN, a neuronal marker, most neurons exhibited increased H3K9/18ac following SAHA or MS-275 treatments in comparison to animals receiving vehicle. These results indicate that histone acetylation in the lumbar spinal cord has been enhanced by intrathecally injected HDACIs and that MS-275 had a comparable effect on histone acetylation as SAHA.
Histone acetylation in the spinal cord after HDACI treatment. Histone acetylation in the lumbar spinal cord of mice receiving i.t. SAHA (25 μg) or MS-275 (0.5 μg) for 30 min was analyzed by immunoblot (A, B) and immunofluorescent histochemistry (C) for antigens indicated. Animals receiving i.t. saline were used as control. Images of the H3K9/18ac signals in the left half of the lumbar spinal cord are shown in the first row in C. Immunosignals of indicated antigens in the superficial dorsal horn are presented in the rest rows in C.
The mechanisms underlying the induction of persistent pain may be different from those for its maintenance. To test whether the spinal HDAC activity could play a different role in these two events, we further studied the effect of SAHA on existing thermal hyperalgesia. SAHA was intrathecally injected in mice that had received intraplantar injection (i.pl.) of CFA for 1, 5 or 24 hr. At these time points, all animals developed peak hyperalgesia before i.t. (Fig. 5). This hypersensitivity was significantly attenuated 30 min after i.t. SAHA in all tested groups (P < 0.05) in comparison to the responses of the same animals before i.t. or to the animals receiving i.t. vehicle.
Attenuation of existing thermal hyperalgesia by HDACI. Mice were treated with CFA i.pl. for indicated time and then intrathecally injected with 25 μg SAHA for 30 min before measurement of thermal response. Control animals were treated with i.t. saline after CFA injection for the same time. Basal line was measured before the CFA injection. Student t-test was used to examine the significance as indicated groups. * P < 0.01.
Since studies above suggest that the activity of class II HDACs in the spinal cord may be critical to induce or maintain CFA-induced hyperalgesia, it is possible that the expression of these enzymes is upregulated in response to tissue damage to support persistent pain hypersensitivity. To test this possibility, using immunoblot analysis, we quantitatively analyzed the levels of different HDACs (HDAC1, 2, 3 in class I and HDAC4, 5, 7, 9 in class IIa) in the lumbar dorsal spinal cord in animals at different time points after receiving CFA. First, we observed for each tested HDAC the bands in the sizes as suggested by manufacturers' instructions. Then, as shown by quantitative analysis in Fig. 6, the expression of members in class I HDACs was found to be stable or be slightly reduced during the time period examined, while those in class IIa HDACs were upregulated significantly to different levels. Importantly, these changes occurred in the early stage following CFA injection, but did not last longer than 24 hrs.
Changes of HDACs' levels in the dorsal spinal cord after CFA injection into the hindpaw. The levels of HDACs in the dorsal spinal cord were analyzed by immunoblot using antibodies specific to each target as indicated. Examples of blots are shown in A and C. Digitally analyzed levels of each HDAC after normalization to β-actin are shown in B for class I HDACs and in D for class IIa HDACs. Mean values plus s.e. are averaged from 4 to 6 animals. Student t-test was used to examine the significance as indicated groups. * P < 0.01; ** P < 0.05.
Discussion
Histone acetylation has been recognized as an important mechanism in epigenetic regulation of gene transcription [14, 15]. One effective approach to test whether histone acetylation impacts a biological event is to inhibit HDAC and then assess the functional output of such an inhibition. In this study, we found that pretreatment of mice with HDACIs, including TSA, SAHA or LAQ824, to target class I and II HDACs in the spinal cord significantly produced a short-term attenuation of CFA-induced thermal hyperalgesia in a dose-dependent manner. A similar reduction was induced by HDACIs specific to class I and IIa HDACs (VPA and 4-PB) [46]. However, application of MS-275 to specifically inhibit class I HDACs failed to attenuate the thermal hyperalgesia, even though it could increase H3 acetylation indicating inhibition of HDAC in the spinal cord (Fig. 4). Our results suggest that a contribution of class I HDACs to the thermal hyperalgesia induced by CFA can be excluded. Then, the inhibited class II HDACs are likely the major players in mediating the attenuation. In addition, analyses of expression of HDACs in the spinal cord following tissue damage caused by CFA revealed that the members in class IIa HDACs underwent upregulation. Therefore, our data suggest that CFA-upregulated class IIa HDACs in the spinal cord may facilitate CFA-induced thermal hyperalgesia, and that the inhibition of class IIa HDACs may be sufficient to attenuate the hyperalgesia.
In a recent report, the second phase of formalin-induced inflammatory pain was reduced by systemically injected MS-275 in the rat [27]. Specifically, MS-275 injected intraperitoneally at 3 mg/kg dosage produced even more inhibition than SAHA at 5 mg/kg dosage. In our studies, however, these two HDACIs i.t. at a similar dose difference, i.e., 0.5 μg of MS-275 vs. 1 μg of SAHA, exhibited significantly different effects on CFA-induced thermal hyperalgesia (Fig. 2). This distinct effect of MS-275 may be explained by following possibilities. First, in our studies, intrathecal injection was used to deliver HDACIs. This approach allows most injected drugs to enter the spinal cord and indeed we observed that the lumbar spinal HDACs were inhibited (Fig. 4). In contrast, systemic administration provides the opportunity for all tissues to receive administered drug and thus to participate in modification of the phenotype changes. These tissues include all structures in the nociceptive pathway from the peripheral to the central nervous system. Therefore, structures other than the spinal cord may be targeted by systemically injected MS-275 and involved in modulation of the pathological pain. Second, the inflammatory pain induced by formalin differs from that induced by CFA in aspects of duration and behavioral changes [58–60], indicating different underlying mechanisms. This notion is supported by a recent report that animals bearing a partial loss-of-function of the HDAC4 gene that belongs to class IIa HDACs exhibited reduced thermal nociception, but no changes in formalin response [44]. Third, in the present study, histone acetylation was examined only for H3K9/18 and H3K9 following MS-275 or SAHA treatment. Changes in other more than 18 lysine residues distributed among at least four different subtypes of histones, i.e., histone 2A, 2B, 3 and 4, may occur [14, 15], but were not examined. Therefore, it is very likely that differential HDAC activities are involved in regulation of different models of persistent pain or that different models of persistent pain may be subjected to distinct epigenetic regulation. In support of this functional difference among HDACs, deletion of the HDAC5 gene, but not the HDAC9 gene, results in a hypersensitive response to chronic cocaine reward or stress in mice [22].
Cell-type distribution of different HDAC isoforms is another factor to be considered for the involvement of specific class HDACs in processing pain signals. Histochemically, distribution of most HDACs in the spinal cord was viewed only by mRNA in situ hybridization provided by the Allen Brain Atlas http://www.brain-map.org/. Based on this database, neurons in the spinal cord express almost all class I and II HDACs. Our data also showed that most neurons (NeuN positive) responded to HDACI treatment by exhibiting more signals of H3K9/18 and H3K9, while much fewer non-neuronal cells showed increased H3 acetylation (Fig. 4C). Taken together, these data suggest that HDAC in spinal neurons may play a major role in persistent pain.
Acetylated histones are major substrates of HDACs and thus modification of HDAC activity inevitably alters gene expression via histone-involved chromatin remodeling. Therefore, gene regulation may be considered as one molecular mechanism underlying the antihyperalgesic effect of HDACIs seen in this study. Genome-wide analyses already revealed that increases in histone acetylation by HDACI even at rest alters mRNA levels of a limited but still significant number of genes either by upregulation or by downregulation [14]. For example, infusion of MS-275 into the nucleus accumbens altered expression of ~435 genes [61]. It is known that expression of a large number of genes in nociceptive pathways impact normal nociception or persistent pain or both [1–3]. Some of those genes may potentiate hypersensitivity while some of them may attenuate hyperactivity to pain signals as evidenced by gene targeting studies in animal models [1]. Some of these genes involved in modification of nociceptive hypersensitivity may be subjected to epigenetic regulation via histone acetylation and thus mediate the antihyperalgesic effect of HDACIs. For example, recently, the expression of the metabotropic glutamate receptor 2 gene in the spinal cord and dorsal root ganglion was found to be upregulated after systemic administration of MS-275 or SAHA, and this upregulation likely mediates the inhibitory effect of these HDACIs on formalin-induced hyperalgesia [27]. Many other genes involved in persistent pain are also regulated by HDACIs. For example, HDAC inhibition increases the promoter activity of the opioid receptor genes [62–64], whose products mediate the analgesic effect of opioid peptides [65]. Another example is the brain-derived neurotrophic factor gene that has been deeply involved in the central sensitization [66, 67], and importantly whose promoters and transcription are heavily regulated by histone acetylation [68–70]. Our results in the present study indicate that inhibition of class II HDACs attenuated thermal hyperalgesia, but not the normal thermal nociceptive response in naïve animals which did not have spinal HDAC induction. On the basis of all observations above, we expect that a large number of genes in the spinal cord undergo expression alteration following HDACI treatment no matter whether animals have been provoked by CFA or not, and the net effect of such expression may favor an attenuation of hypersensitivity to nociceptive stimuli, but the maintenance of normal or unprovoked nociception is not affected. We hypothesize that a pain-alleviating histone acetylation that is sensitive to class IIa HDACs may reside in the spinal cord for the development of persistent pain. The difference between the gene expression profiles resulting from the inhibition of class I HDACs and those following the inhibition of class IIa HDACs is also of interest, in view of their different effect on thermal hyperalgesia. Illustration of this difference in expression profile in the spinal cord may eventually provide insight not only of functional difference of these HDACIs, but also the molecular mechanisms underlying HDACI's antihyperalgesic activity. Our observation that alteration of histone acetylation only impacts persistent pain provides further evidence to support the notion that persistent pain is regulated by epigenetic mechanism [9].
In addition to histone, a few acetylated proteins can be the substrates of HDACs, as well [14, 15, 46, 71] and some of these proteins may mediate the effects of HDCIs on persistent pain via gene regulation or other mechanisms. Recently, these proteins were searched globally and found to include transcription factors, proteins participating in metabolism, cell cycle and signal transduction such as NF-kappa-B-activating kinase [72–74] that involves a pathway regulating inflammatory pain hypersensitivity [7, 75, 76]. In view of the short duration of the HDACI effect, it is possible that acetylation in proteins other than histones was accumulated under the pressure of HDACIs, thus inducing the attenuation of hyperalgesia. This possibility is also supported by three facts. First, class IIa HDAC members are actively exported from the nucleus [14, 15, 77], which provides the opportunity for these enzymes to act on non-nuclear proteins that have been acetylated. Second, class IIa HDACs showed very low activity on acetylated histone used in in vitro test and were proposed to efficiently act on a narrow set of undiscovered substrates [78]. Third, class IIa HDACs interact with different non-histone proteins from those that are associated with class I or class IIb HDACs [14, 79], even in face of our poor understanding of the selectivity of HDACs on acetylated lysines in histone subtypes [13].
Conclusions
The present study demonstrates that inhibition of HDAC in the spinal cord results in a short-term attenuation of thermal hyperalgesia induced by the inflammatory agent CFA and class IIa HDAC may play a major role in this antihyperalgesic effect. This class of HDAC also exhibits upregulation in response to CFA, suggesting that the members in class IIa HDACs are potential targets for attenuation of persistent inflammatory pain. These data indicate that epigenetic regulation in the spinal cord participates in the development of persistent pain and analgesic effects resulting from inhibition of selective HDACs provides a novel target for the development of analgesic drugs.
Methods
Animal and behavior studies. Mice (male, C57BL/6NTac, 6-7 weeks, ~20-22gr, Taconic Farm) were purchased from Taconic. All animals received care in compliance with the Guide for the Care and Use of Laboratory Animals (NIH pub No. 86-23). All experiments involving animals were conducted according to the protocol approved by the Institutional Animal Care and Use Committee at University of Maryland. Intrathecal injection was performed as reported previously [80] with a 5 μl volume to be completed in 5 min. HDACIs were obtained from the following sources (in parenthesis): VPA (Axxora Life Sciences), 4-PB (Enzo Biochem), TSA (Cell Signaling Technologies), SAHA (Cayman Chemical), MS-275 and LAQ824 (Selleck). Saline or 1% DMSO served as vehicle. Control animals received vehicle injection. After the intraplantar injection (i.pl.) of 10 μl of CFA (Sigma, diluted 1:4 with saline) to the left hindpaw, the time of paw withdrawal latency (PWL) to noxious heat was measured from both hindpaws using a system described previously [81]. The injected hindpaw showed edema and erythema indicating inflammation [81]. Intrathecal injection was completed either 30 min before CFA injection or at the selected time after CFA injection. Each experimental group included 8 to 10 animals. All experiments were performed blind for injection and behavioral test. Reversed hyperalgesia was calculated as follows. The PWL differences between the baseline and the treatment of i.t. vehicle followed by i. pl. CFA for a selected time were regarded as the 100% hypersensitivity. The PWL differences between the baseline and the treatment of i.t. tested HDACI followed by i.pl. CFA for the same selected time were regarded the modified hypersensitivity. The differences between the 100% and modified hypersensitivity are the inhibitory effect of tested HDACI.
The effect of intrathecal injection on the spinal target was examined by analyzing histone acetylation 30 min after i.t. selected HDACI by immunohistochemistry and immunoblot using three fresh mice per HDACI per technology. For HDAC expression studies, CFA or saline was injected bilaterally to naïve mice as described above for the time points indicated in Fig. 6. Four to six mice were used for each time point per treatment (CFA or saline) or for naïve animals.
Immunoblot analysis. Immunoblot analysis was carried out as described previously [82]. Naïve or treated animals were sacrificed at selected time points. Spinal cords were quickly pushed out by injecting saline into the spine. The dorsal half of the lumbar spinal cord equal to the L4 to L5 segments were quickly dissected out, frozen on dry ice and saved at -80°C until use. To test the effect of HDACI, the whole lumber spinal cord was collected. Tissue lysates were prepared by homogenizing dissected spinal cord in RIPA buffer plus protease inhibitor cocktail (Roche) in a Teflon-glass tissue grinder followed by 10 min centrifugation at 15,000xg. Lysates (16 μg protein/lane) were fractionated on 7 or 10% polyacrylamide-SDS gel in a glycine-Tris buffer and transferred to a PVDF membrane. Primary antibodies were obtained from Cell Signaling Technology for HDAC1, 2, 3, 4, 5, 7 and Santa Cruz Biotechnology for HDAC9, and were diluted by 1:1,000 in PBST. Histone was examined by an antibody specific to acetylated histone 3 Lys9 (H3K9ac, Upstate/Millipore) and by an antibody specific to pan-H3 (Upstate/Millipore). Enhanced chemical luminescence was used to reveal and quantify signals associated with the specific antigen to be detected. Signals associated with the specific immune complex were analyzed and normalized to β-actin as described previously [82].
Immunohistochemistry. Immunostaining of spinal cord was conducted as described previously [83]. Briefly, mice were perfused with 5% paraformaldehyde/PBS after selected treatments. Spinal cord was dissected out and subjected to post-fixation 5% with paraformaldehyde/PBS and cryostat protection in 30% sucrose/PBS. Transverse sections in 30 μm thick were made of the lumbar section of the spinal cord in a cryostat and mounted directly onto glass slides (Fisher Scientific). Immunostaining was performed on the glass slides with a rabbit antibody against acetylated histone 3 (H3K9/18ac, Upstate/Millipore) and a mouse monoclonal antibody against NeuN (Chemicon/Millipore). Primary antibodies were visualized by anti-rabbit IgG antibody conjugated with Cy3 and anti-mouse IgG antibody labeled with Cy2 (Jackson ImmunoResearch laboratories), respectively.
Data processing: One way ANOVA with post hoc Tukey's test was used for statistical analysis of the data among multiple groups. All data presented in figures are mean values of indicated animals plus standard error.
Abbreviations
- CFA:
-
complete Freund's adjuvant
- HDAC:
-
histone deacetylase
- HDACI:
-
histone deacetylase inhibitor
- H3:
-
histone 3
- VPA:
-
valproic acid
- SAHA:
-
suberoyl anilide bishydroxamide
- 4-PB:
-
4-phenylbutyrate
- TSA:
-
trichostatin
- MS-275:
-
2-aminobenzamides
- i.t.:
-
intrathecal injection
- i.pl.:
-
intraplantar injection.
References
LaCroix-Fralish ML, Ledoux JB, Mogil JS: The Pain Genes Database : An interactive web browser of pain-related transgenic knockout studies. Pain 2007, 131: 3.e1–3.e4. 10.1016/j.pain.2007.04.041
Geranton SM, Morenilla-Palao C, Hunt SP: A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci 2007, 27: 6163–6173. 10.1523/JNEUROSCI.1306-07.2007
Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ: Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci 2007, 27: 8699–8708. 10.1523/JNEUROSCI.2018-07.2007
Rohl T, Kurreck J: RNA interference in pain research. J Neurochem 2006, 99: 371–380. 10.1111/j.1471-4159.2006.04082.x
Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM: DREAM is a critical transcriptional repressor for pain modulation. Cell 2002, 108: 31–43. 10.1016/S0092-8674(01)00629-8
Duric V, McCarson KE: Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol Pain 2007, 3: 32. 10.1186/1744-8069-3-32
Tegeder I, Niederberger E, Schmidt R, Kunz S, Guhring H, Ritzeler O, Michaelis M, Geisslinger G: Specific Inhibition of I{kappa}B Kinase Reduces Hyperalgesia in Inflammatory and Neuropathic Pain Models in Rats. J Neurosci 2004, 24: 1637–1645. 10.1523/JNEUROSCI.3118-03.2004
Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W: Genetic architecture of human pain perception. Trends Genet 2007, 23: 605–613. 10.1016/j.tig.2007.09.004
Feinberg AP: Phenotypic plasticity and the epigenetics of human disease. Nature 2007, 447: 433–440. 10.1038/nature05919
Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC: Epigenetics in the Nervous System. J Neurosci 2008, 28: 11753–11759. 10.1523/JNEUROSCI.3797-08.2008
Henderson I, Jacobsen S: Epigenetic inheritance in plants. Nature 2007, 447: 418–424. 10.1038/nature05917
Mattick JS: Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays 2003, 25: 930–939. 10.1002/bies.10332
Kouzarides T: Chromatin modifications and their function. Cell 2007, 128: 693–705. 10.1016/j.cell.2007.02.005
Haberland M, Montgomery RL, Olson EN: The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 2009, 10: 32–42. 10.1038/nrg2485
Fukuda H, Sano N, Muto S, Horikoshi M: Simple histone acetylation plays a complex role in the regulation of gene expression. Brief Funct Genomic Proteomic 2006, 5: 190–208. 10.1093/bfgp/ell032
Minamiyama M, Katsuno M, Adachi H, Waza M, Sang C, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Sobue G: Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 2004, 13: 1183–1192. 10.1093/hmg/ddh131
Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM: Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 2001, 413: 739–743. 10.1038/35099568
McCampbell A, Taye AA, Whitty L, Penney E, Steffan JS, Fischbeck KH: Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc Natl Acad Sci USA 2001, 98: 15179–15184. 10.1073/pnas.261400698
Thomas EA: Focal nature of neurological disorders necessitates isotype-selective histone deacetylase (HDAC) inhibitors. Mol Neurobiol 2009, 40: 33–45. 10.1007/s12035-009-8067-y
Butler R, Bates GP: Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nat Rev Neurosci 2006, 7: 784–796. 10.1038/nrn1989
Monneret C: Histone deacetylase inhibitors for epigenetic therapy of cancer. Anticancer Drugs 2007, 18: 363–370. 10.1097/CAD.0b013e328012a5db
Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ: Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 2007, 56: 517–529. 10.1016/j.neuron.2007.09.032
Lucio-Eterovic AK, Cortez MA, Valera ET, Motta FJ, Queiroz RG, Machado HR, Carlotti CG, Neder L, Scrideli CA, Tone LG: Differential expression of 12 histone deacetylase (HDAC) genes in astrocytomas and normal brain tissue: class II and IV are hypoexpressed in glioblastomas. BMC Cancer 2008, 8: 243. 10.1186/1471-2407-8-243
Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ: Distribution of histone deacetylases 1–11 in the rat brain. J Mol Neurosci 2007, 31: 47–58. 10.1007/BF02686117
Cunliffe VT: Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development 2004, 131: 2983–2995. 10.1242/dev.01166
Fischer DD, Cai R, Bhatia U, Asselbergs FAM, Song C, Terry R, Trogani N, Widmer R, Atadja P, Cohen D: Isolation and Characterization of a Novel Class II Histone Deacetylase, HDAC10. J Biol Chem 2002, 277: 6656–6666. 10.1074/jbc.M108055200
Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW, Copani A, Nicoletti F: Epigenetic Modulation of mGlu2 Receptors by Histone Deacetylase Inhibitors in the Treatment of Inflammatory Pain. Mol Pharmacol 2009, 75: 1014–1020. 10.1124/mol.108.054346
Sternberg WF, Ridgway CG: Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav 2003, 78: 375–383. 10.1016/S0031-9384(03)00015-5
Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA: Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol 2002, 282: G307–316.
Bernardi M, Genedani S, Tagliavini S, Bertolini A: Effects on long-term sensitivity to pain and morphine of stress induced in the newborn rat by pain or manipulation. Physiol Behav 1986, 37: 827–831. 10.1016/0031-9384(86)90191-5
Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS: Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain 2004, 110: 588–596. 10.1016/j.pain.2004.04.006
Rodier PM: Environmental Causes of Central Nervous System Maldevelopment. Pediatrics 2004, 113: 1076–1083.
Fejer R, Hartvigsen J, Kyvik KO: Heritability of neck pain: a population-based study of 33 794 Danish twins. Rheumatology 2006, 45: 589–594. 10.1093/rheumatology/kei224
Bellos F, Mahlknecht U: Valproic acid and all-trans retinoic acid: meta-analysis of a palliative treatment regimen in AML and MDS patients. Onkologie 2008, 31: 629–633. 10.1159/000160599
Kuendgen A, Gattermann N: Valproic acid for the treatment of myeloid malignancies. Cancer 2007, 110: 943–954. 10.1002/cncr.22891
Bialer M, Yagen B: Valproic Acid: second generation. Neurotherapeutics 2007, 4: 130–137. 10.1016/j.nurt.2006.11.007
Vinik A: CLINICAL REVIEW: Use of antiepileptic drugs in the treatment of chronic painful diabetic neuropathy. J Clin Endocrinol Metab 2005, 90: 4936–4945. 10.1210/jc.2004-2376
Lussier D, Huskey AG, Portenoy RK: Adjuvant analgesics in cancer pain management. Oncologist 2004, 9: 571–591. 10.1634/theoncologist.9-5-571
Adcock IM, Tsaprouni L, Bhavsar P, Ito K: Epigenetic regulation of airway inflammation. Curr Opin Immunol 2007, 19: 694–700. 10.1016/j.coi.2007.07.016
Butler KV, Kozikowski AP: Chemical origins of isoform selectivity in histone deacetylase inhibitors. Curr Pharm Des 2008, 14: 505–528. 10.2174/138161208784480180
Glauben R, Sonnenberg E, Zeitz M, Siegmund B: HDAC inhibitors in models of inflammation-related tumorigenesis. Cancer Lett 2009, 280: 154–159. 10.1016/j.canlet.2008.11.019
Kuendgen A, Gattermann N: Valproic acid for the treatment of myeloid malignancies. Cancer 2007, 110: 943–954. 10.1002/cncr.22891
Johannessen CU, Johannessen SI: Valproate: past, present, and future. CNS Drug Rev 2003, 9: 199–216. 10.1111/j.1527-3458.2003.tb00249.x
Rajan I, Savelieva KV, Ye G-L, Wang C-y, Malbari MM, Friddle C, Lanthorn TH, Zhang W: Loss of the Putative Catalytic Domain of HDAC4 Leads to Reduced Thermal Nociception and Seizures while Allowing Normal Bone Development. PLoS ONE 2009, 4: e6612. 10.1371/journal.pone.0006612
Uchida H, Ma L, Ueda H: Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci 2010, 30: 4806–4814. 10.1523/JNEUROSCI.5541-09.2010
Dokmanovic M, Clarke C, Marks PA: Histone Deacetylase Inhibitors: Overview and Perspectives. Mol Cancer Res 2007, 5: 981–989. 10.1158/1541-7786.MCR-07-0324
Banner LR, Patterson PH, Allchorne A, Poole S, Woolf CJ: Leukemia Inhibitory Factor Is an Anti-Inflammatory and Analgesic Cytokine. J Neurosci 1998, 18: 5456–5462.
Winkler I, Blotnik S, Shimshoni J, Yagen B, Devor M, Bialer M: Efficacy of antiepileptic isomers of valproic acid and valpromide in a rat model of neuropathic pain. Br J Pharmacol 2005, 146: 198–208. 10.1038/sj.bjp.0706310
Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T: Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 2001, 20: 6969–6978. 10.1093/emboj/20.24.6969
Balasubramanian S, Verner E, Buggy JJ: Isoform-specific histone deacetylase inhibitors: the next step? Cancer Lett 2009, 280: 211–221. 10.1016/j.canlet.2009.02.013
Ellis L, Atadja PW, Johnstone RW: Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther 2009, 8: 1409–1420. 10.1158/1535-7163.MCT-08-0860
Eot-Houllier G, Fulcrand G, Magnaghi-Jaulin L, Jaulin C: Histone deacetylase inhibitors and genomic instability. Cancer Lett 2009, 274: 169–176. 10.1016/j.canlet.2008.06.005
Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M: Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 2008, 409: 581–589. 10.1042/BJ20070779
Bhalla KN: Epigenetic and Chromatin Modifiers As Targeted Therapy of Hematologic Malignancies. J Clin Oncol 2005, 23: 3971–3993. 10.1200/JCO.2005.16.600
Eikel D, Hoffmann K, Zoll K, Lampen A, Nau H: S -2-pentyl-4-pentynoic hydroxamic acid and its metablite s -2-pentyl-4--pentynoic acid in the NMRI-exencephaly-mouse model: pharmacokinetic profiles, teratogenic effects, and histone deacetylase inhibition abilities of further valproic acid hydroxamates and amides. Drug Metab Dispos 2006, 34: 612–620. 10.1124/dmd.105.008078
Simonini MV, Camargo LM, Dong E, Maloku E, Veldic M, Costa E, Guidotti A: The benzamide MS-275 is a potent, long-lasting brain region-selective inhibitor of histone deacetylases. Proc Natl Acad Sci USA 2006, 103: 1587–1592. 10.1073/pnas.0510341103
Hahnen E, Ilker Y, Eyüpoglu IY, Brichta L, Haastert K, Tränkle C, Siebzehnrübl FA, Riessland M, Hölker I, Claus P, Romstöck J, Buslei R, Wirth B, Blümcke I: In vitro and ex vivo evaluation of second-generation histone deacetylase inhibitors for the treatment of spinal muscular atrophy. J Neurochem 2006, 98: 193–202. 10.1111/j.1471-4159.2006.03868.x
Tjølsen A, Berge O-G, Hunskaar S, Rosland JH, Hole K: The formalin test: an evaluation of the method. Pain 1992, 51: 5–17. 10.1016/0304-3959(92)90003-T
Coderre T, Melzack R: The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci 1992, 12: 3671–3675.
Ren K, Dubner R: Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol 1996, 76: 3025–3037.
Covington HEI, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJI, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ: Antidepressant Actions of Histone Deacetylase Inhibitors. J Neurosci 2009, 29: 11451–11460. 10.1523/JNEUROSCI.1758-09.2009
Hwang CK, Kim CS, Kim DK, Law P-Y, Wei L-N, Loh HH: Up-regulation of the mu opioid receptor gene is mediated through chromatin remodeling and transcriptional factors in differentiated neuronal cells. Mol Pharmacol 2010. published on-line by April 12, 2010
Bi J, Hu X, Loh HH, Wei L-N: Regulation of Mouse {kappa} Opioid Receptor Gene Expression by Retinoids. J Neurosci 2001, 21: 1590–1599.
Wang G, Liu T, Wei L-N, Law P-Y, Loh HH: DNA Methylation-Related Chromatin Modification in the Regulation of Mouse {delta}-Opioid Receptor Gene. Mol Pharmacol 2005, 67: 2032–2039. 10.1124/mol.105.011056
Kieffer BL, Gavériaux-Ruff C: Exploring the opioid system by gene knockout. Prog Neurobiol 2002, 66: 285–306. 10.1016/S0301-0082(02)00008-4
Biggs J, Lu V, Stebbing M, Balasubramanyan S, Smith P: Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol Pain 2010, 6: 44. 10.1186/1744-8069-6-44
Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W: Molecular Depletion of Descending Serotonin Unmasks Its Novel Facilitatory Role in the Development of Persistent Pain. J Neurosci 2010, 30: 8624–8636. 10.1523/JNEUROSCI.5389-09.2010
Huang Y, Doherty JJ, Dingledine R: Altered Histone Acetylation at Glutamate Receptor 2 and Brain-Derived Neurotrophic Factor Genes Is an Early Event Triggered by Status Epilepticus. J Neurosci 2002, 22: 8422–8428.
Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M: Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 2007, 14: 268–276. 10.1101/lm.500907
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ: Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 2006, 9: 519–525. 10.1038/nn1659
Mariadason JM: HDACs and HDAC inhibitors in colon cancer. Epigenetics 2008, 3: 28–37. 10.4161/epi.3.1.5736
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan K-L: Regulation of Cellular Metabolism by Protein Lysine Acetylation. Science 2010, 327: 1000–1004. 10.1126/science.1179689
Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning Z-B, Zeng R, Xiong Y, Guan K-L, Zhao S, Zhao G-P: Acetylation of Metabolic Enzymes Coordinates Carbon Source Utilization and Metabolic Flux. Science 2010, 327: 1004–1007. 10.1126/science.1179687
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M: Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325: 834–840. 10.1126/science.1175371
Niederberger E, Geisslinger G: The IKK-NF-{kappa}B pathway: a source for novel molecular drug targets in pain therapy? FASEB J 2008, 22: 3432–3442. 10.1096/fj.08-109355
Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ: Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci 2004, 19: 3375–81. 10.1111/j.0953-816X.2004.03441.x
McKinsey TA, Zhang CL, Olson EN: Identification of a Signal-Responsive Nuclear Export Sequence in Class II Histone Deacetylases. Mol Cell Biol 2001, 21: 6312–6321. 10.1128/MCB.21.18.6312-6321.2001
Lachner M, O'Sullivan RJ, Jenuwein T: An epigenetic road map for histone lysine methylation. J Cell Sci 2003, 116: 2117–2124. 10.1242/jcs.00493
Yang X-J, Gregoire S: Class II Histone Deacetylases: from Sequence to Function, Regulation, and Clinical Implication. Mol Cell Biol 2005, 25: 2873–2884. 10.1128/MCB.25.8.2873-2884.2005
Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, Muglia LJ, Zhuo M: Oxytocin mediates stress-induced analgesia in adult mice. J Physiol 2002, 540: 593–606. 10.1113/jphysiol.2001.013492
Iadarola MJ, Brady LS, Draisci G, Dubner R: Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain 1988, 35: 313–326. 10.1016/0304-3959(88)90141-8
Bai G, Zhuang ZY, Liu AG, Chai YF, Hoffman PW: The role of the RE1 element in activation of the NR1 promoter during neuronal differentiation. J Neurochem 2003, 86: 992–1005. 10.1046/j.1471-4159.2003.01922.x
Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S, Bai G, Zhang X: Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest 2005, 115: 3104–3116. 10.1172/JCI25509
Acknowledgements
Authors wish to thank members in Drs. Ren and Dubner's laboratories for technical help, and for Dr. Feng Wei's help in the intrathecal injection of mice. This work is supported partially by NIH grant NS059028 and NS060735.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GB initiated and designed this project, analyzed data and drafted the manuscript. GB also performed immunoblot analysis and participated in animal studies for blind experiments. DW and SZ conducted animal studies and immunohistochemical experiments. KR and RD participated in experimental design, data analysis and the finalization of the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bai, G., Wei, D., Zou, S. et al. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain 6, 51 (2010). https://doi.org/10.1186/1744-8069-6-51
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-6-51