Abstract
Background
Glutamate and oxidative stress play important roles after subarachnoid hemorrhage (SAH). The ability to modulate glutamate transporter 1 (GLT-1) and the antioxidative effect of rosiglitazone have been demonstrated. We investigated the neuroprotective effect of rosiglitazone after SAH.
Methods
SAH was induced by double blood injection. The rats were randomly divided into sham, SAH + vehicle, and SAH + rosiglitazone groups and treated with dimethyl sulfoxide, dimethyl sulfoxide, and 6 mg/kg of rosiglitazone, respectively, at 2 and 12 h after SAH induction and then daily for 6 days. Cerebrospinal fluid dialysates were collected 30 min before SAH induction and then daily for 7 days for glutamate measurement. Mortality, body weight, and neurological scores were also measured daily. On day 7 after SAH, the wall thickness and the perimeter of the basilar artery (BA), neuron variability, GLT-1 levels, glial fibrillary acidic protein (GFAP) expression and activity, and malondialdehyde, superoxide dismutase, and catalase activities were also evaluated.
Results
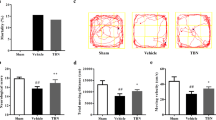
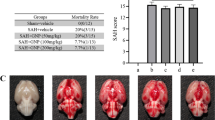
Rosiglitazone improved survival (relative risk = 0.325) and neurological functions and reduced neuronal degeneration (5.7 ± 0.8 vs. 10.0 ± 0.9; P < 0.001) compared with the SAH + vehicle group. Rosiglitazone also lowered glutamate levels by 43.5-fold and upregulated GLT-1 expression by 1.5-fold and astrocyte activity by 1.8-fold compared with the SAH + vehicle group. The increase in BA wall thickness was significantly attenuated by rosiglitazone, whereas the perimeter of the BA was increased. In addition, rosiglitazone abated the 1.9-fold increase in malondialdehyde levels and the 1.6-fold increase in catalase activity after SAH.
Conclusion
Rosiglitazone reduced SAH mortality, neurological deficits, body weight loss, GFAP loss, and cerebral vasospasm by preventing the neurotoxicity induced by glutamate and oxidative stress.








Similar content being viewed by others
References
Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–96.
Kuo CP, Lu CH, Wen LL, et al. Neuroprotective effect of curcumin in an experimental rat model of subarachnoid hemorrhage. Anesthesiology. 2011;115:1229–38.
Wu CT, Wen LL, Wong CS, et al. Temporal changes in glutamate, glutamate transporters, basilar arteries wall thickness, and neuronal variability in an experimental rat model of subarachnoid hemorrhage. Anesth Analg. 2011;112:666–73.
Germano A, Caffo M, Angileri FF, et al. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–44.
Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14.
Swanson RA, Farrell K, Stein BA. Astrocyte energetics, function, and death under conditions of incomplete ischemia: a mechanism of glial death in the penumbra. Glia. 1997;21:142–53.
van Landeghem FK, Weiss T, Oehmichen M, von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J Neurotrauma. 2006;23:1518–28.
Lopez-Redondo F, Nakajima K, Honda S, Kohsaka S. Glutamate transporter GLT-1 is highly expressed in activated microglia following facial nerve axotomy. Brain Res Mol Brain Res. 2000;76:429–35.
Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–86.
Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Delayed cell death related to acute cerebral blood flow changes following subarachnoid hemorrhage in the rat brain. J Neurosurg. 2005;102:1046–54.
Sabri M, Kawashima A, Ai J, Macdonald RL. Neuronal and astrocytic apoptosis after subarachnoid hemorrhage: a possible cause for poor prognosis. Brain Res. 2008;1238:163–71.
Murakami K, Koide M, Dumont TM, Russell SR, Tranmer BI, Wellman GC. Subarachnoid hemorrhage induces gliosis and increased expression of the pro-inflammatory cytokine high mobility group box 1 protein. Transl Stroke Res. 2011;2:72–9.
Raghubir R, Verma R, Samuel SS, Raza S, Haq W, Katti SB. Anti-stroke profile of thiazolidin-4-one derivatives in focal cerebral ischemia model in rat. Chem Biol Drug Des. 2011;78:445–53.
Collino M, Aragno M, Mastrocola R, et al. Modulation of the oxidative stress and inflammatory response by PPAR-gamma agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol. 2006;530:70–80.
Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–26.
Wu Y, Zhao XD, Zhuang Z, et al. Peroxisome proliferator-activated receptor gamma agonist rosiglitazone attenuates oxyhemoglobin-induced Toll-like receptor 4 expression in vascular smooth muscle cells. Brain Res. 2010;1322:102–8.
Wu Y, Tang K, Huang RQ, et al. Therapeutic potential of peroxisome proliferator-activated receptor gamma agonist rosiglitazone in cerebral vasospasm after a rat experimental subarachnoid hemorrhage model. J Neurol Sci. 2011;305:85–91.
Kuo CP, Wen LL, Chen CM, et al. Attenuation of neurological injury with early baicalein treatment following subarachnoid hemorrhage in rats. J Neurosurg. 2013;119:1028–37.
Sung CS, Wen ZH, Chang WK, et al. Intrathecal interleukin-1beta administration induces thermal hyperalgesia by activating inducible nitric oxide synthase expression in the rat spinal cord. Brain Res. 2004;1015:145–53.
Prunell GF, Mathiesen T, Svendgaard NA. A new experimental model in rats for study of the pathophysiology of subarachnoid hemorrhage. NeuroReport. 2002;13:2553–6.
Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–7.
Meguro T, Clower BR, Carpenter R, Parent AD, Zhang JH. Improved rat model for cerebral vasospasm studies. Neurol Res. 2001;23:761–6.
Seckin H, Yigitkanli K, Besalti O, et al. Lamotrigine attenuates cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Surg Neurol. 2008;70:344–51 (discussion 51).
Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–33 (discussion 33-5).
Rothoerl RD, Schebesch KM, Kubitza M, Woertgen C, Brawanski A, Pina AL. ICAM-1 and VCAM-1 expression following aneurysmal subarachnoid hemorrhage and their possible role in the pathophysiology of subsequent ischemic deficits. Cerebrovasc Dis. 2006;22:143–9.
Davalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28:708–10.
Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–81.
Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–9.
Garcia-Bueno B, Caso JR, Perez-Nievas BG, Lorenzo P, Leza JC. Effects of peroxisome proliferator-activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacology. 2007;32:1251–60.
Han F, Shioda N, Moriguchi S, Qin ZH, Fukunaga K. Downregulation of glutamate transporters is associated with elevation in extracellular glutamate concentration following rat microsphere embolism. Neurosci Lett. 2008;430:275–80.
Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage: changes in incidence and case fatality from 1985 through 2000. Stroke. 2004;35:2059–63.
Law RE, Goetze S, Xi XP, et al. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–8.
Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ Res. 2008;102:283–94.
Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci. 2007;28:244–9.
Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161:113–22.
Fatehi-Hassanabad Z, Tasker RA. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation confers functional neuroprotection in global ischemia. Neurotox Res. 2011;19:462–71.
Sullivan SM, Bjorkman ST, Miller SM, Colditz PB, Pow DV. Morphological changes in white matter astrocytes in response to hypoxia/ischemia in the neonatal pig. Brain Res. 2010;1319:164–74.
Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–7.
Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–34.
Adelson PD, Jenkins LW, Hamilton RL, Robichaud P, Tran MP, Kochanek PM. Histopathologic response of the immature rat to diffuse traumatic brain injury. J Neurotrauma. 2001;18:967–76.
Sayan-Ozacmak H, Ozacmak VH, Barut F, Jakubowska-Dogru E. Neuroprotective efficacy of the peroxisome proliferator-activated receptor-gamma ligand in chronic cerebral hypoperfusion. Curr Neurovasc Res. 2011;8:190–9.
Cowley TR, O’Sullivan J, Blau C, et al. Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in LTP. Neurobiol Aging. 2012;33:162–75.
Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol Dis. 2005;18:476–83.
Chen JC, Hsu-Chou H, Lu JL, et al. Down-regulation of the glial glutamate transporter GLT-1 in rat hippocampus and striatum and its modulation by a group III metabotropic glutamate receptor antagonist following transient global forebrain ischemia. Neuropharmacology. 2005;49:703–14.
Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41.
Jeon H, Ai J, Sabri M, et al. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009;10:103.
Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–72.
Luo Y, Yin W, Signore AP, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–48.
Sheu WH, Chuang HC, Cheng SM, Lee MR, Chou CC, Cheng FC. Microdialysis combined blood sampling technique for the determination of rosiglitazone and glucose in brain and blood of gerbils subjected to cerebral ischemia. J Pharm Biomed Anal. 2011;54:759–64.
Acknowledgments
The authors thank Meei-Shyuan Lee, PhD, Professor, School of Public Health, National Defense Medical Center, Taipei, Taiwan, Republic of China, for statistic consultation. The grant was supported by National Science Council (NSC 97-2314-B-016-005-MY2) and Tri-Service General Hospital (TSGH-C100-114) of Taiwan, Republic of China.
Conflict of interest
The other authors have no financial or institutional conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, BF., Kuo, CY., Wen, LL. et al. Rosiglitazone Attenuates Cerebral Vasospasm and Provides Neuroprotection in an Experimental Rat Model of Subarachnoid Hemorrhage. Neurocrit Care 21, 316–331 (2014). https://doi.org/10.1007/s12028-014-0010-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0010-z