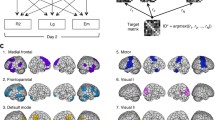
Abstract
Using resting-state functional magnetic resonance imaging (rs-fMRI) to study functional connectivity is of great importance to understand normal development and function as well as a host of neurological and psychiatric disorders. Seed-based analysis is one of the most widely used rs-fMRI analysis methods. Here we describe a freely available large scale functional connectivity data mining software package called Advanced Connectivity Analysis (ACA). ACA enables large-scale seed-based analysis and brain-behavior analysis. It can seamlessly examine a large number of seed regions with minimal user input. ACA has a brain-behavior analysis component to delineate associations among imaging biomarkers and one or more behavioral variables. We demonstrate applications of ACA to rs-fMRI data sets from a study of autism.


Similar content being viewed by others
References
Anderson, J. S., Druzgal, T. J., Froehlich, A., DuBray, M. B., Lange, N., Alexander, A. L., et al. (2011). Decreased interhemispheric functional connectivity in autism. Cerebral Cortex, 21(5), 1134–1146.
Baker, J. T., Holmes, A. J., Masters, G. A., Yeo, B. T., Krienen, F., Buckner, R. L., et al. (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry, 71(2), 109–118.
Chen, R., & Herskovits, E. H. (2005). Graphical-model based morphometric analysis. IEEE Transactions on Medical Imaging, 24(10), 1237–1248.
Chen, R., & Herskovits, E. H. (2007a). Clinical diagnosis based on Bayesian classification of functional magnetic-resonance data. Neuroinformatics, 5(3), 178–188.
Chen, R., & Herskovits, E. H. (2007b). Graphical-model-based multivariate analysis of functional magnetic resonance data. NeuroImage, 35, 635–647.
Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173.
Craddock, R. C., Jbabdi, S., Yan, C. G., Vogelstein, J. T., Castellanos, F. X., Di Martino, A., et al. (2013). Imaging human connectomes at the macroscale. Nature Methods, 10(6), 524–539.
Di Martino, A., Yan, C. G., Li, Q., Denio, E., Castellanos, F. X., Alaerts, K., et al. (2014). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular Psychiatry, 19(6), 659–667.
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience, 8(9), 700–711.
Friston, K. J., Frith, C. D., Liddle, P. F., & Frackowiak, R. S. (1993). Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow and Metabolism, 13(1), 5–14.
Hallquist, M. N., Hwang, K., & Luna, B. (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 82, 208–225.
Heckerman, D., & Chickering, D. M. (1995). Learning Bayesian networks: the combination of knowledge and statistical data. Machine Learning, 20-197.
Homack, S., Lee, D., & Riccio, C. A. (2005). Test review: delis-Kaplan executive function system. Journal of Clinical and Experimental Neuropsychology, 27(5), 599–609.
Hong, L. E., Gu, H., Yang, Y., Ross, T. J., Salmeron, B. J., Buchholz, B., et al. (2009). Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Archives of General Psychiatry, 66(4), 431–441.
Hong, L. E., Hodgkinson, C. A., Yang, Y., Sampath, H., Ross, T. J., Buchholz, B., et al. (2010). A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proceedings of the National Academy of Sciences of the United States of America, 107(30), 13509–13514.
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). FSL NeuroImage, 62, 782–790.
Just, M. A., Cherkassky, V. L., Keller, T. A., & Minshew, N. J. (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain, 127(Pt 8), 1811–1821.
Koller, D., & Friedman, N. (2009). Probabilistic graphical models: principles and techniques. Cambridge: The MIT Press.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685.
Lord, C., Risi, S., Lambrecht, L., Cook Jr., E. H., Leventhal, B. L., DiLavore, P. C., et al. (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223.
McCarthy, H., Skokauskas, N., Mulligan, A., Donohoe, G., Mullins, D., Kelly, J., et al. (2013). Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry, 70(12), 1329–1337.
Monk, C. S., Peltier, S. J., Wiggins, J. L., Weng, S. J., Carrasco, M., Risi, S., et al. (2009). Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage, 47(2), 764–772.
NIH (2014). Brain research through advancing innovative neurotechnologies (BRAIN) working group report to the advisory committee to the director, NIH
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154.
Rosner, B. (2010). Fundamentals of biostatistics (Seventh ed., ). Boston: Brooks/Cole.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155.
Song, X. W., Dong, Z. Y., Long, X. Y., Li, S. F., Zuo, X. N., Zhu, C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One, 6(9), e25031.
Tang, L., Ge, Y., Sodickson, D. K., Miles, L., Zhou, Y., Reaume, J., et al. (2011). Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology, 260(3), 831–840.
Turner, K. C., Frost, L., Linsenbardt, D., McIlroy, J. R., & Muller, R. A. (2006). Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and Brain Functions, 2, 34.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–289.
Vissers, M. E., Cohen, M. X., & Geurts, H. M. (2012). Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and Biobehavioral Reviews, 36(1), 604–625.
von dem Hagen, E. A., Stoyanova, R. S., Baron-Cohen, S., & Calder, A. J. (2013). Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social Cognitive and Affective Neuroscience, 8(6), 694–701.
Wang, Q., Chen, R., JaJa, J., Jin, Y., Hong, L. E., & Herskovits, E. H. (2015). Connectivity-based brain parcellation: a connectivity-based atlas for schizophrenia research. Neuroinformatics. doi:10.1007/s12021-015-9280-7.
Weng, S. J., Wiggins, J. L., Peltier, S. J., Carrasco, M., Risi, S., Lord, C., et al. (2010). Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research, 1313, 202–214.
Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141.
Woodward, N. D., Rogers, B., & Heckers, S. (2011). Functional resting-state networks are differentially affected in schizophrenia. Schizophrenia Research, 130(1–3), 86–93.
Acknowledgments
This research has been funded by the Center for Health-related Informatics and Bioimaging (CHIB) under Maryland’s MPower Initiative.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
An Example Study
In this example, we demonstrate how to implement a large-scale seed-based analysis using ACA. This example is included in the ACA package as a test data set. We analyzed imaging and behavior data from the Nathan Kline Institute (NKI)/Rockland sample (downloaded from http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). The NKI/Rockland sample includes 204 normal subjects. For each subject, a ten minute rs-fMRI scan was provided. In this example, our primary outcome variable was age. The validation behavior variable was the score of the Delis-Kaplan Executive Function sorting test (confirmed correct sorts), which is a problem solving task (Homack et al. 2005). This behavior variable has been implicated in general overall executive function which is associated with age.
The T1 and rs-fMRI data were preprocessed using ACA. For each seed region, the rsFC maps for all subjects were saved in a directory. For seed-based analysis, the first step was to create a project file. We created a CSV file to define the imaging data, consisting of three columns: the first column was the subject name, the second column was the primary outcome variable, and the third column represented whether or not this subject was excluded. Then we created a CSV file, which included the behavior variables. The first column of the behavior variable file was the subject ID; and the subsequent variables were behavior variables. For both the imaging and behavior CSV files, we created an associated data type file. If a variable was continuous, its type was coded as ‘1’. If a variable was categorical, its type was coded as ‘2’.
When the imaging and behavior CSV files were complete, we conducted the analysis. The user interface of ACA is simple. We opened a Linux/Unix terminal, went to the project home directory which included the raw rsFC maps, and the imaging and behavior CSV file, and then typed commands. For step by step analysis, we first typed aca_sba_ana_1a.sh to prepare data and generate study masks. Then we typed aca_sba_ana_2a.sh, aca_sba_ana_2b.sh, or aca_sba_ana_2c.sh for biomarker detection. aca_sba_ana_2a.sh is for biomarker detection based on regression, aca_sba_ana_2b.sh is based on ANOVA, and aca_sba_ana_2c.sh is GAMMA-based biomarker detection. In this example, the primary outcome variable was a continuous variable; therefore, we used aca_sba_ana_2a.sh for biomarker detection. After biomarker detection, we typed aca_sba_ana_3a.sh for brain-behavior analysis. Then we typed aca_sba_summarize_1.sh to summarize the detected biomarkers.
Rights and permissions
About this article
Cite this article
Chen, R., Nixon, E. & Herskovits, E. Advanced Connectivity Analysis (ACA): a Large Scale Functional Connectivity Data Mining Environment. Neuroinform 14, 191–199 (2016). https://doi.org/10.1007/s12021-015-9290-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12021-015-9290-5