Abstract
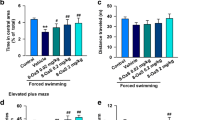
Epidemiological studies demonstrate that pain frequently occurs comorbid with depression. Gentiopicroside (Gent) is a secoiridoid compound isolated from Gentiana lutea that exhibits analgesic properties and inhibits the expression of GluN2B-containing N-methyl-d-aspartate (NMDA) receptors in the anterior cingulate cortex of mice. However, the effects of Gent on the reserpine-induced pain/depression dyad and its underlying mechanisms are unclear. Reserpine administration (1 mg/kg subcutaneous daily for 3 days) caused a significant decrease in the nociceptive threshold as evidenced by the reduced paw withdrawal latency in response to a radiant heat source and mechanical allodynia. Behavioral detection indicated a significant increase in immobility time during a forced swim test, as well as decreased time in the central area and total travel distance in an open field test. Furthermore, reserpinized animals exhibited increased oxidative stress. Systemic Gent administration dose-dependently ameliorated the behavioral deficits associated with reserpine-induced pain/depression dyad. At the same time, the decrease in biogenic amine levels (norepinephrine, dopamine, and serotonin) was integrated with the increase in caspase-3 levels and GluN2B-containing NMDA receptors in the amygdala of the reserpine-injected mice. Gent significantly reversed the changes in the levels of biogenic amines, caspase-3, and GluN2B-containing NMDA receptors in amygdala. However, Gent did not affect the expression of GluN2A-containing NMDA receptors. The inhibitory effects of Gent on oxidative stress were occluded by simultaneous treatment of GluN2B receptors antagonist Ro25-6981. Our study provides strong evidence that Gent inhibits reserpine-induced pain/depression dyad by downregulating GluN2B receptors in the amygdala.






Similar content being viewed by others
Abbreviations
- 5-HT:
-
Serotonin
- BLA:
-
Basolateral amygdala
- CAT:
-
Catalase
- CeA:
-
Central nucleus amygdala
- CNS:
-
Central nervous system
- DA:
-
Dopamine
- Gent:
-
Gentiopicroside
- LA:
-
Lateral amygdala
- MDA:
-
Malondialdehyde
- NE:
-
Norepinephrine
- NMDA:
-
N-methyl-d-aspartate
- OP:
-
Open field
- PWL:
-
Paw withdrawal latency
- TST:
-
Tail suspension test
References
Amaral, O. B., & Roesler, R. (2008). Targeting the NMDA receptor for fear-related disorders. Recent Patents on CNS Drug Discovery, 3(3), 166–178.
Arora, V., Kuhad, A., Tiwari, V., & Chopra, K. (2011). Curcumin ameliorates reserpine-induced pain-depression dyad: Behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology, 36(10), 1570–1581.
Bair, M. J., Robinson, R. L., Katon, W., & Kroenke, K. (2003). Depression and pain comorbidity: A literature review. Archives of Internal Medicine, 163(20), 2433–2445.
Bar, K. J., Wagner, G., Koschke, M., Boettger, S., Boettger, M. K., Schlosser, R., et al. (2007). Increased prefrontal activation during pain perception in major depression. Biological Psychiatry, 62(11), 1281–1287.
Blackburn-Munro, G., & Blackburn-Munro, R. E. (2001). Chronic pain, chronic stress and depression: Coincidence or consequence? Journal of Neuroendocrinology, 13(12), 1009–1023.
Blier, P., & Abbott, F. V. (2001). Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. Journal of Psychiatry and Neuroscience, 26(1), 37–43.
Cates, L. N., Roberts, A. J., Huitron-Resendiz, S., & Hedlund, P. B. (2013). Effects of lurasidone in behavioral models of depression. Role of the 5-HT receptor subtype. Neuropharmacology, 70C, 211–217.
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M., & Yaksh, T. L. (1994). Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods, 53(1), 55–63.
Chen, L., Liu, J. C., Zhang, X. N., Guo, Y. Y., Xu, Z. H., Cao, W., et al. (2008). Down-regulation of NR2B receptors partially contributes to analgesic effects of gentiopicroside in persistent inflammatory pain. Neuropharmacology, 54(8), 1175–1181.
Ehrlich, I., Humeau, Y., Grenier, F., Ciocchi, S., Herry, C., & Luthi, A. (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron, 62(6), 757–771.
Elhwuegi, A. S. (2004). Central monoamines and their role in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28(3), 435–451.
Feng, B., Liu, J. C., Zhang, J., Ozaki, K., Guo, Y. Y., Yi, D. H., et al. (2013). Anxiolytic actions of motilin in the basolateral amygdala. Molecular Neurobiology, 47(3), 892–902.
Guo, Y. Y., Liu, S. B., Cui, G. B., Ma, L., Feng, B., Xing, J. H., et al. (2012). Acute stress induces down-regulation of large-conductance Ca2+ -activated potassium channels in the lateral amygdala. Journal of Physiology, 590(Pt 4), 875–886.
Hamilton, J. P., Siemer, M., & Gotlib, I. H. (2008). Amygdala volume in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry, 13(11), 993–1000.
Hase, K., Li, J., Basnet, P., Xiong, Q., Takamura, S., Namba, T., et al. (1997). Hepatoprotective principles of Swertia japonica Makino on D-galactosamine/lipopolysaccharide-induced liver injury in mice. Chemical and Pharmaceutical Bulletin (Tokyo), 45(11), 1823–1827.
Heydarpour, P., Salehi-Sadaghiani, M., Javadi-Paydar, M., Rahimian, R., Fakhfouri, G., Khosravi, M., et al. (2013). Estradiol reduces depressive-like behavior through inhibiting nitric oxide/cyclic GMP pathway in ovariectomized mice. Hormones and Behavior, 63(2), 361–369.
Hu, J., Wang, Z., Guo, Y. Y., Zhang, X. N., Xu, Z. H., Liu, S. B., et al. (2009). A role of periaqueductal grey NR2B-containing NMDA receptor in mediating persistent inflammatory pain. Mol Pain, 5, 71.
Karolewicz, B., Szebeni, K., Gilmore, T., Maciag, D., Stockmeier, C. A., & Ordway, G. A. (2009). Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. International Journal of Neuropsychopharmacology, 12(2), 143–153.
Lakhan, S. E., Caro, M., & Hadzimichalis, N. (2013). NMDA receptor activity in neuropsychiatric disorders. Front Psychiatry, 4, 52.
Li, N., Lee, B., Liu, R. J., Banasr, M., Dwyer, J. M., Iwata, M., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329(5994), 959–964.
Liu, S. B., Ma, L., Guo, H. J., Feng, B., Guo, Y. Y., Li, X. Q., et al. (2012a). Gentiopicroside attenuates morphine rewarding effect through downregulation of GluN2B receptors in nucleus accumbens. CNS Neuroscience & Therapeutics, 18(8), 652–658.
Liu, Y., Wong, T. P., Aarts, M., Rooyakkers, A., Liu, L., Lai, T. W., et al. (2007). NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. Journal of Neuroscience, 27(11), 2846–2857.
Liu, L., Wong, T. P., Pozza, M. F., Lingenhoehl, K., Wang, Y., Sheng, M., et al. (2004). Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science, 304(5673), 1021–1024.
Liu, S. B., Zhang, N., Guo, Y. Y., Zhao, R., Shi, T. Y., Feng, S. F., et al. (2012b). G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. Journal of Neuroscience, 32(14), 4887–4900.
Lohr, J. B., Kuczenski, R., & Niculescu, A. B. (2003). Oxidative mechanisms and tardive dyskinesia. CNS Drugs, 17(1), 47–62.
Marks, D. M., Shah, M. J., Patkar, A. A., Masand, P. S., Park, G. Y., & Pae, C. U. (2009). Serotonin-norepinephrine reuptake inhibitors for pain control: Premise and promise. Current Neuropharmacology, 7(4), 331–336.
Molosh, A. I., Sajdyk, T. J., Truitt, W. A., Zhu, W., Oxford, G. S., & Shekhar, A. (2013). NPY Y(1) Receptors differentially modulate GABA(A) and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology, 38, 1352.
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B., & Seeburg, P. H. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron, 12(3), 529–540.
Nash, J. E., Hill, M. P., & Brotchie, J. M. (1999). Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Experimental Neurology, 155(1), 42–48.
Ozturk, N., Baser, K. H., Aydin, S., Ozturk, Y., & Calis, I. (2002). Effects of Gentiana lutea ssp. symphyandra on the central nervous system in mice. Phytotherapy Research, 16(7), 627–631.
Ozturk, N., Korkmaz, S., Ozturk, Y., & Baser, K. H. (2006). Effects of gentiopicroside, sweroside and swertiamarine, secoiridoids from gentian (Gentiana lutea ssp. symphyandra), on cultured chicken embryonic fibroblasts. Planta Medica, 72(4), 289–294.
Paoletti, P. (2011). Molecular basis of NMDA receptor functional diversity. European Journal of Neuroscience, 33(8), 1351–1365.
Paoletti, P., Bellone, C., & Zhou, Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience, 14(6), 383–400.
Potvin, S., Grignon, S., & Marchand, S. (2009). Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse (New York, N. Y.), 63(5), 390–402.
Rainnie, D. G., Bergeron, R., Sajdyk, T. J., Patil, M., Gehlert, D. R., & Shekhar, A. (2004). Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. Journal of Neuroscience, 24(14), 3471–3479.
Romano, J. M., & Turner, J. A. (1985). Chronic pain and depression: does the evidence support a relationship? Psychological Bulletin, 97(1), 18–34.
Rouwette, T., Vanelderen, P., Roubos, E. W., Kozicz, T., & Vissers, K. (2012). The amygdala, a relay station for switching on and off pain. European Journal of Pain, 16(6), 782–792.
Russell, I. J., Vaeroy, H., Javors, M., & Nyberg, F. (1992). Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis and Rheumatism, 35(5), 550–556.
Sacher, J., Neumann, J., Funfstuck, T., Soliman, A., Villringer, A., & Schroeter, M. L. (2012). Mapping the depressed brain: A meta-analysis of structural and functional alterations in major depressive disorder. Journal of Affective Disorders, 140(2), 142–148.
Senol, F. S., Tuzun, C. Y., Toker, G., & Orhan, I. E. (2012). An in vitro perspective to cholinesterase inhibitory and antioxidant activity of five Gentiana species and Gentianella caucasea. International Journal of Food Sciences and Nutrition, 63(7), 802–812.
Smith, G. R. (1992). The epidemiology and treatment of depression when it coexists with somatoform disorders, somatization, or pain. General Hospital Psychiatry, 14(4), 265–272.
Stanika, R. I., Pivovarova, N. B., Brantner, C. A., Watts, C. A., Winters, C. A., & Andrews, S. B. (2009). Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proceedings of the National Academy of Sciences, 106, 9854–9859.
Tasan, R. O., Nguyen, N. K., Weger, S., Sartori, S. B., Singewald, N., Heilbronn, R., et al. (2010). The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. Journal of Neuroscience, 30(18), 6282–6290.
Tye, K. M., Prakash, R., Kim, S. Y., Fenno, L. E., Grosenick, L., Zarabi, H., et al. (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature, 471(7338), 358–362.
Wang, S. H., de Oliveira Alvares, L., & Nader, K. (2009). Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nature Neuroscience, 12(7), 905–912.
Wang, D., Yuan, X., Liu, T., Liu, L., Hu, Y., Wang, Z., et al. (2012). Neuroprotective activity of lavender oil on transient focal cerebral ischemia in mice. Molecules, 17(8), 9803–9817.
Wu, L. J., Toyoda, H., Zhao, M. G., Lee, Y. S., Tang, J., Ko, S. W., et al. (2005). Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. Journal of Neuroscience, 25(48), 11107–11116.
Wu, L. J., & Zhuo, M. (2009). Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics, 6(4), 693–702.
Wyllie, D. J., Livesey, M. R., & Hardingham, G. E. (2013). Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology, 74, 4–17.
Xu, Y., Zhang, L., Shao, T., Ruan, L., Wang, L., Sun, J., et al. (2013). Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: Behavioral and neurobiological analyses. Metabolic Brain Disease, 28(4), 571–583.
Zarate, C. A, Jr, Singh, J. B., Carlson, P. J., Brutsche, N. E., Ameli, R., Luckenbaugh, D. A., et al. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry, 63(8), 856–864.
Zhao, M. G., Toyoda, H., Lee, Y. S., Wu, L. J., Ko, S. W., Zhang, X. H., et al. (2005). Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron, 47(6), 859–872.
Acknowledgments
This research was supported by National Natural Science Foundation of China, Nos. 31070923, 31271126, 31271144, 2011ZXJ09106-01C, and 2011KTCL03-12.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shui-bing Liu, Rong Zhao, and Xu-sheng Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Sb., Zhao, R., Li, Xs. et al. Attenuation of Reserpine-Induced Pain/Depression Dyad by Gentiopicroside Through Downregulation of GluN2B Receptors in the Amygdala of Mice. Neuromol Med 16, 350–359 (2014). https://doi.org/10.1007/s12017-013-8280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8280-8