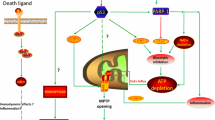
Abstract
Peroxynitrite is a powerful oxidant, formed from the reaction of nitric oxide and superoxide. It is known to interact and modify different biological molecules such as DNA, lipids and proteins leading to alterations in their structure and functions. These events elicit various cellular responses, including cell signaling, causing oxidative damage and committing cells to apoptosis or necrosis. This review discusses nitrosative stress-induced modification in the DNA molecule that results in the formation of 8-nitroguanine and 8-oxoguanine, and its role in disease conditions. Different approaches of cell death, such as necrosis and apoptosis, are modulated by cellular high-energy species, such as ATP and NAD+. High concentrations of peroxynitrite are known to cause necrosis, whereas low concentrations lead to apoptosis. Any damage to DNA activates cellular DNA repair machinery, like poly(ADP-ribose) polymerase (PARP). PARP-1, an isoform of PARP, is a DNA nick-sensing enzyme that becomes activated upon sensing DNA breakage and triggers the cleavage of NAD+ into nicotinamide and ADP-ribose and polymerizes the latter on nuclear acceptor proteins. Peroxynitrite-induced hyperactivation of PARP causes depletion of NAD+ and ATP culminating cell dysfunction, necrosis or apoptosis. This mechanistic pathway is implicated in the pathogenesis of a variety of diseases, including circulatory shock (which is characterized by cellular hypoxia triggered by systemic altered perfusion and tissue oxygen utilization leading end-organ dysfunction), sepsis and inflammation, injuries of the lung and the intestine. The cytotoxic effects of peroxynitrite centering on the participation of PARP-1 and ADP-ribose in previously stated diseases have also been discussed in this review.



Similar content being viewed by others
Abbreviations
- dG:
-
Deoxyguanosine
- PARP:
-
Poly(ADP-ribose) polymerase
- 8-NitroG:
-
8-Nitroguanine
- 8-OxoG:
-
8-Oxoguanine
- NI:
-
5-Guanidino-4-nitroimidazole
- Iz:
-
2,5-Diamino-4H-imidazol-4-one
- Oz:
-
2,2,4-Triamino-5(2H)-oxazolone
- AAP:
-
Acetaminophen
- 5-AIQ:
-
5-Aminoisoquinoline
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- 3AB:
-
3-Aminobenzamide
- IR:
-
Ischemia–reperfusion
- DNBS:
-
Dinitrobenzene sulfonic acid
- TNBS:
-
Trinitrobenzene sulfonic acid
- iNOS:
-
Inducible nitric oxide synthase
- TNFα:
-
Tumor necrosis factor alpha
- MODS:
-
Multiple organ dysfunction syndrome
- HMGB1:
-
High-mobility group protein 1
- AIF:
-
Apoptosis-inducing factor
- PARG:
-
PAR glycohydrolase
- NAD+ :
-
Nicotinamide adenine dinucleotide (oxidized)
References
Denicola, A., Souza, J. M., & Radi, R. (1998). Diffusion of peroxynitrite across erythrocyte membranes. Proceedings of the National Academy of Sciences of the United States of America, 95, 3566–3571.
Radi, R., Beckman, J. S., Bush, K. M., & Freeman, B. A. (1991). Peroxynitrite oxidation of sulfhydryls: The cytotoxic potential of superoxide and nitric oxide. Journal of Biological Chemistry, 266, 4244–4250.
Bartesaghi, S., Valez, V., Trujillo, M., Peluffo, G., Romero, N., Zhang, H., et al. (2006). Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-t-BOC-l-tyrosine tert-butyl ester. Biochemistry, 45, 6813–6825.
Quijano, C., Alvarez, B., Gatti, R., Augusto, O., & Radi, R. (1997). Pathways of peroxynitrite oxidation of thiol groups. Biochemical Journal, 322, 167–173.
Bonini, M. G., & Augusto, O. (2001). Carbon dioxide stimulates the production of thiyl, sulfinyl, and disulfide radical anion from thiol oxidation by peroxynitrite. Journal of Biological Chemistry, 276, 9749–9754.
Salgo, M. G., Bermudez, E., Squadrito, G. L., & Pryor, W. A. (1995). Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes. Archives of Biochemistry and Biophysics, 322, 500–505.
Szabó, C., & Ohshima, H. (1997). DNA damage induced by peroxynitrite: Subsequent biological effects. Nitric Oxide, 1, 373–385.
Burney, S., Niles, J. C., Dedon, P. C., & Tannenbaum, S. R. (1999). DNA damage in deoxynucleosides and oligonucleotides treated with peroxynitrite. Chemical Research in Toxicology, 12, 513–520.
Niles, J. C., Wishnok, J. S., & Tannenbaum, S. R. (2006). Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: Structures and mechanisms of product formation. Nitric Oxide, 14, 109–121.
Kennedy, L. J., Moore, K., Jr., Caulfield, J. L., Tannenbaum, S. R., & Dedon, P. C. (1997). Quantitation of 8-oxoguanine and strand breaks produced by four oxidizing agents. Chemical Research in Toxicology, 10, 386–392.
Radi, R. (2004). NO, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences of the United States of America, 101, 4003–4008.
Villa, L. M., Salas, E., Darley-Usmar, V. M., Radomski, M. W., & Moncada, S. (1994). Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proceedings of the National Academy of Sciences of the United States of America, 91, 12383–12387.
Rubbo, H., Radi, R., Trujillo, M., Telleri, R., Kalyanaraman, B., Barnes, S., et al. (1994). Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. Journal of Biological Chemistry, 269, 26066–26075.
Violi, F., Marino, R., Milite, M. T., & Loffredo, L. (1999). NO and its role in lipid peroxidation. Diabetes Metabolism Research and Reviews, 15, 283–288.
Wright, M. M., Schopfer, F. J., Baker, P. R., Vidyasagar, V., Powell, P., Chumley, P., et al. (2006). Fatty acid transduction of nitric oxide signaling: Nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proceedings of the National Academy of Sciences of the United States of America, 103, 4299–4304.
Milstien, S., & Katusic, Z. (1999). Oxidation of tetrahydrobiopterin by peroxynitrite: Implications for vascular endothelial function. Biochemical and Biophysical Research Communications, 263, 681–684.
Ischiropoulos, H., Zhu, L., & Beckman, J. S. (1992). Peroxynitrite formation from macrophage-derived nitric oxide. Archives of Biochemistry and Biophysics, 298, 446–451.
Lewis, R. S., Tamir, S., Tannenbaum, S. R., & Deen, W. M. (1995). Kinetic analysis of the fate of nitric oxide synthesized by macrophages in vitro. Journal of Biological Chemistry, 270, 29350–29355.
Faulkner, K. M., Liochev, S. I., & Fridowich, I. (1994). Stable Mn(III) prophyrins mimic cuperoxide dismutase in vitro and substitute for it in vivo. Journal of Biological Chemistry, 269, 23471–23476.
Yermilov, V., Rubio, J., Becchi, M., Friesen, M. D., Pignatelli, B., & Ohshima, H. (1995). Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis, 16, 2045–2050.
Koppenol, W. H., Moreno, J. J., Pryor, W. A., Ischiropoulos, H., & Beckman, J. S. (1992). Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chemical Research in Toxicology, 5, 834–842.
Rubio, J., Yermilov, V., & Ohshima, H. (1996). DNA damage induced by peroxynitrite: Formation of 8-nitroguanine and base propenals. In Moncada, S., Stamler, J., Gross, S., & Higgs, E. A. (Eds.), The biology of nitric oxide (p. 34). London: Portland Press Proceedings, part 5.
Yermilov, V., Yoshie, Y., Rubio, J., & Ohshima, H. (1996). Effects of carbon dioxide/bicarbonate on induction of DNA single-strand breaks and formation of 8-nitroguanine, 8-oxo-guanine and base-propenal mediated by peroxynitrite. FEBS Letters, 399, 67–70.
Yermilov, V., Rubio, J., & Ohshima, H. (1995). Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Letters, 376, 207–210.
Spencer, J. P., Wong, J., Jenner, A., Aruoma, O. I., Cross, C. E., & Halliwell, B. (1996). Base modification and strand breakage in isolated calf thymus DNA and in DNA from human skin epidermal keratinocytes exposed to peroxynitrite or 3-morpholinosydnonimine. Chemical Research in Toxicology, 9, 1152–1158.
Epe, B., Ballmaier, D., Roussyn, I., Briviba, K., & Sies, H. (1996). DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acids Research, 24, 4105–4110.
Beckman, J. S., Beckman, T. W., Chen, J., Marshall, P. A., & Freeman, B. A. (1990). Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of Sciences of the United States of America, 87, 1620–1624.
Beckman, J. S., Estevez, A. G., Crow, J. P., & Barbeito, L. (2001). Superoxide dismutase and the death of motoneurons in ALS. Trends in Neurosciences, 24, S15–S20.
Beckman, J. S., Carson, M., Smith, C. D., & Koppenol, W. H. (1993). ALS, SOD and peroxynitrite. Nature, 364, 584.
Beckman, J. S., & Koppenol, W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. American Journal of Physiology Cell Physiology, 271, C1424–C1437.
Messmer, U. K., Reimer, D. M., Reed, J. C., & Brune, B. (1996). Nitric oxide induced poly(ADP-ribose) polymerase cleavage in RAS 264.7 macrophage apoptosis is blocked by Bcl-2. FEBS Letters, 384, 162–166.
Riquelme, P. T., Burzio, L. O., & Koide, S. S. (1979). ADP ribosylation of rat liver lysine-rich histone in vitro. Journal of Biological Chemistry, 254, 3018–3028.
Suzuki, H., Quesada, P., Farina, B., & Leone, E. (1986). In vitro poly(ADP-ribosyl)ation of seminal ribonuclease. Journal of Biological Chemistry, 261, 6048–6055.
Lautier, D., Lagueux, J., Thiboldeau, J., Menard, L., & Poirier, G. G. (1993). Molecular and biochemical features of poly(ADP-ribose)metabolism. Molecular and Cellular Biochemistry, 122, 171–193.
Min, W., & Wang, Z. Q. (2009). Poly(ADP-ribose) glycohydrolase (PARG) and its therapeutic potential. Frontiers in Bioscience (Landmark Edition), 14, 1619–1626.
Otto, H., Reche, P. A., Bazan, F., Dittmar, K., Haag, F., & Koch-Nolte, F. (2005). In silico characterization of the family of PARP-like poly(ADP-ribosyl)transferases (pARTs). BMC Genomics, 6, 139.
Hottiger, M. O., Hassa, P. O., Lüscher, B., Schüler, H., & Koch-Nolte, F. (2010). Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends in Biochemical Sciences, 35, 208–219.
Dantzer, F., Amé, J. C., Schreiber, V., Nakamura, J., Ménissier-de Murcia, J., & de Murcia, G. (2006). Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods in Enzymology, 409, 493–510.
Hassa, P. O., & Hottiger, M. O. (2008). The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Frontiers Biosciences, 13, 3046–3082.
Jagtap, P., & Szabó, C. (2005). Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nature Reviews Drug Discovery, 4, 421–440.
Beneke, S. (2008). Poly(ADP-ribose) polymerase activity in different pathologies—The link to inflammation and infarction. Experimental Gerontology, 43, 605–614.
Virág, L., & Szabó, C. (2002). The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacological Reviews, 54, 375–429.
Szabó, C., Ischiropoulos, H., & Radi, R. (2007). Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nature Reviews Drug Discovery, 6, 662–680.
Pacher, P., Beckman, J. S., & Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiological Reviews, 87, 315–324.
Aguilar-Quesada, R., Muñoz-Gámez, J. A., Martín-Oliva, D., Peralta-Leal, A., Quiles-Pérez, R., Rodríguez-Vargas, J. M., et al. (2007). Modulation of transcription by PARP-1: Consequences in carcinogenesis and inflammation. Current Medicinal Chemistry, 14, 1179–1187.
Susin, S. A., Lorenzo, H. K., Zamzami, N., Marzo, I., Snow, B. E., Brothers, G. M., et al. (1999). Molecular characterization of mitochondrial apoptosis-inducing factor. Nature, 397, 441–446.
MacMillan-Crow, L. A., & Thompson, J. A. (1999). Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Archives of Biochemistry and Biophysics, 366, 82–88.
Radi, R., Cassina, A., & Hodara, R. (2002). Nitric oxide and peroxynitrite interactions with mitochondria. Biological Chemistry, 383, 401–409.
Radi, R., Cassina, A., Hodara, R., Quijano, C., & Castro, L. (2002). Peroxynitrite reactions and formation in mitochondria. Free Radical Biology and Medicine, 33, 1451–1464.
Castro, L., Rodriguez, M., & Radi, R. (1994). Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. Journal of Biological Chemistry, 269, 29409–29415.
Han, D., Canali, R., Garcia, J., Aguilera, R., Gallaher, T. K., & Cadenas, E. (2005). Sites and mechanisms of aconitase inactivation by peroxynitrite: Modulation by citrate and glutathione. Biochemistry, 44, 11986–11996.
Stachowiak, O., Dolder, M., Wallimann, T., & Richter, C. (1998). Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. Journal of Biological Chemistry, 273, 16694–16699.
Kumar, A., & Parrillo, J. E. (2001). Shock: Classification, pathophysiology, and approach to management. In J. D. Parillo & R. Dellinger (Eds.), Critical care medicine: Principles of diagnosis and management in the adult (2nd ed., pp. 371–420). St. Louis: Mosby.
Weil, M. H., & Shubin, H. (1971). Proposed reclassification of shock states with special reference to distributive defects. Advances in Experimental Medicine and Biology, 23, 13–23.
Levy, B., Collin, S., Sennoun, N., Ducrocq, N., Kimmoun, A., Asfar, P., et al. (2010). Vascular hyporesponsiveness to vasopressors in septic shock: From bench to bedside. Intensive Care Medicine, 36, 2019–2029.
Hollenberg, S. M. (2009). Inotrope and vasopressor therapy of septic shock. Critical Care Clinics, 25, 781–802.
Szabó, C., & Modis, K. (2010). Pathophysiological roles of peroxynitrite in circulatory shock. Shock, 34(Suppl 1), 4–14.
Pacher, P., & Szabó, C. (2008). Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. American Journal of Pathology, 173, 2–13.
Esposito, E., & Cuzzocrea, S. (2009). Role of nitroso radicals as drug targets in circulatory shock. British Journal of Pharmacology, 157, 494–508.
Jagtap, P., Soriano, F. G., Virág, L., Liaudet, L., Mabley, J., Szabó, E., et al. (2002). Novel phenanthridinone inhibitors of poly (adenosine 5′-diphosphate-ribose) synthetase: Potent cytoprotective and antishock agents. Critical Care Medicine, 30, 1071–1082.
Goldfarb, R. D., Marton, A., Szabó, E., Virág, L., Salzman, A. L., Glock, D., et al. (2002). Protective effect of a novel, potent inhibitor of poly(adenosine 5′-diphosphate-ribose) synthetase in a porcine model of severe bacterial sepsis. Critical Care Medicine, 30, 974–980.
Soriano, F. G., Liaudet, L., Szabó, E., Virág, L., Mabley, J. G., Pacher, P., et al. (2002). Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock, 17, 286–292.
Liaudet, L., Soriano, F. G., Szabó, E., Virág, L., Mabley, J. G., Salzman, A. L., et al. (2000). Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proceedings of the National Academy of Sciences of the United States of America, 97, 10203–10208.
Li, J., Li, W., Altura, B. T., & Altura, B. M. (2004). Peroxynitrite-induced relaxation in isolated canine cerebral arteries and mechanisms of action. Toxicology and Applied Pharmacology, 196, 176–182.
Ohashi, M., Faraci, F., & Heistad, D. (2005). Peroxynitrite hyperpolarizes smooth muscle and relaxes internal carotid artery in rabbit via ATP-sensitive K+ channels. American Journal of Physiology Heart and Circulatory Physiology, 289, H2244–H2250.
Cena, J. J., Lalu, M. M., Cho, W. J., Chow, A. K., Bagdan, M. L., Daniel, E. E., et al. (2010). Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. American Journal of Physiology Heart and Circulatory Physiology, 298, H45–H51.
Grover, A. K., Samson, S. E., Robinson, S., & Kwan, C. Y. (2003). Effects of peroxynitrite on sarcoplasmic reticulum Ca2+ pump in pig coronary artery smooth muscle. American Journal of Physiology. Cell Physiology, 284, C294–C301.
Martínez-Caro, L., Nin, N., Sánchez-Rodríguez, C., Ferruelo, A., El Assar, M., de Paula, M., et al. (2015). Inhibition of nitro-oxidative stress attenuates pulmonary and systemic injury induced by high-tidal volume mechanical ventilation. Shock, 44, 36–43.
Szabó, C., Cuzzocrea, S., Zingarelli, B., O’Connor, M., & Salzman, A. L. (1997). Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly(ADP-ribose) synthetase by peroxynitrite. Journal of Clinical Investigation, 100, 723–735.
Merx, M. W., & Weber, C. (2007). Sepsis and the heart. Circulation, 116, 793–802.
Rudiger, A., & Singer, M. (2007). Mechanisms of sepsis-induced cardiac dysfunction. Critical Care Medicine, 35, 1599–1608.
Hunter, J. D., & Doddi, M. (2010). Sepsis and the heart. British Journal of Anaesthesia, 104, 3–11.
Levrand, S., Vannay-Bouchiche, C., Pesse, B., Pacher, P., Feihl, F., Waeber, B., et al. (2006). Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radical Biology and Medicine, 41, 886–895.
Pacher, P., Liaudet, L., Mabley, J. G., Cziráki, A., Haskó, G., & Szabó, C. (2006). Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. International Journal of Molecular Medicine, 17, 369–375.
Soriano, F. G., Nogueira, A. C., Caldini, E. G., Lins, M. H., Teixeira, A. C., Cappi, S. B., et al. (2006). Potential role of poly(adenosine 5′-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Critical Care Medicine, 34, 1073–1079.
Lokuta, A. J., Maertz, N. A., Meethal, S. V., Potter, K. T., Kamp, T. J., Valdivia, H. H., et al. (2005). Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation, 111, 988–995.
Snook, J. H., Li, J., Helmke, B. P., & Guilford, W. H. (2008). Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radical Biology and Medicine, 44, 14–23.
Borbély, A., Tóth, A., Edes, I., Virág, L., Papp, J. G., Varró, A., et al. (2005). Peroxynitrite-induced alpha-actinin nitration and contractile alterations in isolated human myocardial cells. Cardiovascular Research, 67, 225–233.
Mihm, M. J., Coyle, C. M., Schanbacher, B. L., Weinstein, D. M., & Bauer, J. A. (2001). Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovascular Research, 49, 798–807.
León, H., Baczkó, I., Sawicki, G., Light, P. E., & Schulz, R. (2008). Inhibition of matrix metalloproteinases prevents peroxynitrite-induced contractile dysfunction in the isolated cardiac myocyte. British Journal of Pharmacology, 153, 676–683.
Polewicz, D., Cadete, V. J., Doroszko, A., Hunter, B. E., Sawicka, J., Szczesna-Cordary, D., et al. (2011). Ischemia induced peroxynitrite dependent modifications of cardiomyocyte MLC1 increases its degradation by MMP-2 leading to contractile dysfunction. Journal of Cellular and Molecular Medicine, 15, 1136–1147.
Loukili, N., Rosenblatt-Velin, N., Rolli, J., Levrand, S., Feihl, F., Waeber, B., et al. (2010). Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner. Journal of Biological Chemistry, 285, 15746–15752.
Pesse, B., Levrand, S., Feihl, F., Waeber, B., Gavillet, B., Pacher, P., et al. (2005). Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. Journal of Molecular and Cellular Cardiology, 38, 765–775.
Loukili, N., Rosenblatt-Velin, N., Li, J., Clerc, S., Pacher, P., Feihl, F., et al. (2011). Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovascular Research, 89, 586–594.
Gerö, D., & Szabó, C. (2008). Poly(ADP-ribose) polymerase: A new therapeutic target? Current Opinion in Anesthesiology, 21, 111–121.
Salvemini, D., Riley, D. P., Lennon, P. J., Wang, Z. Q., Currie, M. G., Macarthur, H., et al. (1999). Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. British Journal of Pharmacology, 127, 685–692.
Vaschetto, R., Kuiper, J. W., Musters, R. J., Eringa, E. C., Della Corte, F., Murthy, K., et al. (2010). Renal hypoperfusion and impaired endothelium-dependent vasodilation in an animal model of VILI: The role of the peroxynitrite-PARP pathway. Critical Care, 14, R45.
Maybauer, D. M., Maybauer, M. O., Szabó, C., Cox, R. A., Westphal, M., Kiss, L., et al. (2011). The peroxynitrite catalyst WW-85 improves pulmonary function in ovine septic shock. Shock, 35, 148–155.
Hauschildt, S., Scheipers, P., Bessler, W., Schwarz, K., Ullmer, A., Flad, H. D., et al. (1997). Role of ADP-ribosylation in activated monocytes/macrophages. Advances in Experimental Medicine and Biology, 419, 249–252.
Szabó, C., Lim, L. H., Cuzzocrea, S., Getting, S. J., Zingarelli, B., Flower, R. J., et al. (1997). Inhibition of poly(ADP-ribose) synthetase attenuates neutrophil recruitment and exerts anti-inflammatory effects. Journal of Experimental Medicine, 186, 1041–1049.
Szabó, C., Zingarelli, B., & Salzman, A. L. (1996). Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circulation Research, 78, 1051–1063.
Iványi, Z., Hauser, B., Pittner, A., Asfar, P., Vassilev, D., Nalos, M., et al. (2003). Systemic and hepatosplanchnic hemodynamic and metabolic effects of the PARP inhibitor PJ34 during hyperdynamic porcine endotoxemia. Shock, 19, 415–421.
Stehr, A., Ploner, F., Tugtekin, I., Matejovic, M., Theisen, M., Zülke, C., et al. (2003). Effect of combining nicotinamide as a PARS-inhibitor with selective iNOS blockade during porcine endotoxemia. Intensive Care Medicine, 29, 995–1002.
Boulos, M., Astiz, M. E., Barua, R. S., & Osman, M. (2003). Impaired mitochondrial function induced by serum from septic shock patients is attenuated by inhibition of nitric oxide synthase and poly(ADP-ribose) synthase. Critical Care Medicine, 31, 353–358.
Szabó, C. (2007). Poly(ADP-ribose) polymerase activation and circulatory shock. In Novartis Foundation symposium (Vol. 280, pp. 92–103) (discussion 103–107, 160–164).
Hauschildt, S., Scheipers, P., Bessler, W. G., & Mülsch, A. (1992). Induction of nitric oxide synthase in L929 cells by tumour-necrosis factor alpha is prevented by inhibitors of poly(ADP-ribose) polymerase. Biochemical Journal, 288, 255–260.
Haddad, M., Rhinn, H., Bloquel, C., Coqueran, B., Szabó, C., Plotkine, M., et al. (2006). Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. British Journal of Pharmacology, 149, 23–30.
Jijon, H. B., Churchill, T., Malfair, D., Wessler, A., Jewell, L. D., Parsons, H. G., et al. (2000). Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. American Journal of Physiology Gastrointestinal and Liver Physiology, 279, G641–G651.
Mazzon, E., Dugo, L., Li, J. H., Di Paola, R., Genovese, T., Caputi, A. P., et al. (2002). GPI 6150, a PARP inhibitor, reduces the colon injury caused by dinitrobenzene sulfonic acid in the rat. Biochemical Pharmacology, 64, 327–337.
Su, C. F., Liu, D. D., Kao, S. J., & Chen, H. I. (2007). Nicotinamide abrogates acute lung injury caused by ischaemia/reperfusion. European Respiratory Journal, 30, 199–204.
Zingarelli, B., Salzman, A. L., & Szabó, C. (1998). Genetic disruption of poly(ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circulation Research, 83, 85–94.
Szabó, C. (2006). Poly(ADP-ribose) polymerase activation by reactive nitrogen species—relevance for the pathogenesis of inflammation. Nitric Oxide, 14, 169–179.
Zingarelli, B., O’Connor, M., & Hake, P. W. (2003). Inhibitors of poly(ADP-ribose) polymerase modulate signal transduction pathways in colitis. European Journal of Pharmacology, 469, 183–194.
Di Paola, R., Mazzon, E., Xu, W., Genovese, T., Ferrraris, D., Muià, C., et al. (2005). Treatment with PARP-1 inhibitors, GPI 15427 or GPI 16539, ameliorates intestinal damage in rat models of colitis and shock. European Journal of Pharmacology, 527(1–3), 163–171.
Cuzzocrea, S., Zingarelli, B., Costantino, G., Szabó, A., Salzman, A. L., Caputi, A. P., et al. (1997). Beneficial effects of 3-aminobenzamide, an inhibitor of poly(ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. British Journal of Pharmacology, 121, 1065–1074.
Chen, C. F., Wang, D., Lin, H. I., Leu, F. J., Shen, C. Y., & Chou, C. C. (2007). Ischemia/reperfusion of the liver induces heart injury in rats. Transplantation Proceedings, 39, 855–857.
Szijártó, A., Batmunkh, E., Hahn, O., Mihály, Z., Kreiss, A., Kiss, A., et al. (2007). Effect of PJ-34 PARP-inhibitor on rat liver microcirculation and antioxidant status. Journal of Surgical Research, 142, 72–80.
Khandoga, A., Biberthaler, P., Enders, G., & Krombach, F. (2004). 5-Aminoisoquinolinone, a novel inhibitor of poly(adenosine disphosphate-ribose) polymerase, reduces microvascular liver injury but not mortality rate after hepatic ischemia-reperfusion. Critical Care Medicine, 32, 472–477.
Cover, C., Fickert, P., Knight, T. R., Fuchsbichler, A., Farhood, A., Trauner, M., et al. (2005). Pathophysiological role of poly(ADP-ribose) polymerase (PARP) activation during acetaminophen-induced liver cell necrosis in mice. Toxicological Sciences, 84, 201–208.
Yamamoto, K., Tsukidate, K., & Farber, J. L. (1993). Differing effects of the inhibition of poly(ADP-ribose) polymerase on the course of oxidative cell injury in hepatocytes and fibroblasts. Biochemical Pharmacology, 46, 483–491.
Acknowledgements
One of the authors B. U. I. is thankful to UGC-MANF for financial support as Senior Research Fellow. Assistance from the Institution (AMU) as well as infrastructural support from DST-FIST to the department is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Islam, B.U., Habib, S., Ali, S.A. et al. Role of Peroxynitrite-Induced Activation of Poly(ADP-Ribose) Polymerase (PARP) in Circulatory Shock and Related Pathological Conditions. Cardiovasc Toxicol 17, 373–383 (2017). https://doi.org/10.1007/s12012-016-9394-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-016-9394-7