Abstract
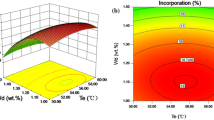
Phosphatidylcholine enriched with docosahexaenoic acid (DHA) was successfully produced by phospholipase A1-catalysed acidolysis. Reverse micelles were firstly selected as the reaction system to avoid the process of immobilization and to ensure the efficient catalysis of phospholipase A1. Parameters were optimized for a high incorporation of phosphatidylcholine enriched with DHA (DHA-PC). A response-surface design with four factors, including the water content, enzyme loading, pH and substrate-mass ratio were used to evaluate the influence of the major factors and to predict the optimal reaction conditions. The results indicated that the optimal reaction conditions for the production of DHA-PC were a water content of 0.4%, an enzyme loading of 40%, pH 6.92 and a substrate-mass ratio of 2.13. Under these optimized conditions, 20.90% of DHA content in DHA-PC was obtained.





Similar content being viewed by others
References
Kris-Etherton, P. M., Harris, W. S., & Appel, L. J. (2002). Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation, 106, 2747–2757.
Simopoulos, A. P. (2002). Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition, 21, 495–505.
Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., Bennett, D. A., Wilson, R. S., Aggarwal, N., & Schneider, J. (2003). Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Archives of Neurology, 60, 940–946.
Das, U. N. (2008). Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease—but how and why? Prostaglandins, Leukotrienes and Essential Fatty Acids, 78, 11–19.
Ren, L. J., Zhuang, X. Y., Chen, S. L., Ji, X. J., & Huang, H. (2015). Introduction of ω-3 Desaturase obviously changed the fatty acid profile and sterol content of Schizochytrium sp. Journal of Agricultural and Food Chemistry, 63, 9770–9776.
Galli, C., Sirtori, C. R., Mosconi, C., Medini, L., Gianfranceschi, G., Vaccarino, V., & Scolastico, C. (1992). Prolonged retention of doubly labeled phosphatidylcholine in human plasma and erythrocytes after oral administration. Lipids, 27, 1005–1012.
Ikeda, I., Sasaki, E., Yasunami, H., Nomiyama, S., Nakayama, M., Sugano, M., Imaizumi, K., & Yazawa, K. (1995). Digestion and lymphatic transport of eicosapentaenoic and docosahexaenoic acids given in the form of triacylglycerol, free acid and ethyl ester in rats. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1259, 297–304.
Dyerberg, J., Madsen, P., Møller, J. M., Aardestrup, I., & Schmidt, E. B. (2010). Bioavailability of marine n-3 fatty acid formulations. Prostaglandins, Leukotrienes and Essential Fatty Acids, 83, 137–141.
Schuchardt, J. P., & Hahn, A. (2013). Bioavailability of long-chain omega-3 fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA), 89, 1–8.
Bhattacharya, S., & Biswas, J. (2009). Understanding membranes through the molecular design of lipids. Langmuir, 26, 4642–4654.
Kidd, P., & Head, K. (2005). A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos®). Alternative Medicine Review, 10, 193–203.
Reddy, J., Vijeeta, T., Karuna, M., Rao, B., & Prasad, R. (2005). Lipase-catalyzed preparation of palmitic and stearic acid-rich phosphatidylcholine. Journal of the American Oil Chemists’ Society, 82, 727–730.
Bi, Y. H., Duan, Z. Q., Li, X. Q., Wang, Z. Y., & Zhao, X. R. (2015). Introducing Biobased ionic liquids as the nonaqueous media for enzymatic synthesis of phosphatidylserine. Journal of Agricultural and Food Chemistry, 63, 1558–1561.
Rosseto, R., & Hajdu, J. (2014). Synthesis of phospholipids on a glyceric acid scaffold: design and preparation of phospholipase A2 specific substrates. Tetrahedron, 70, 3155–3165.
Zhao, T. T., Kim, B. H., Garcia, H. S., Kim, Y., & Kim, I. H. (2014). Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chemistry, 157, 132–140.
Sakai, K., Okuyama, H., Yura, J., Takeyama, H., Shinagawa, N., Tsuruga, N., Kato, K., Miura, K., Kawase, K., & Tsujimura, T. (1992). Composition and turnover of phospholipids and neutral lipids in human breast cancer and reference tissues. Carcinogenesis, 13, 579–584.
Pepping, J. (1999). Phosphatidylserine. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists, 56(2038), 2043–2034.
Hiratsuka, S., Ishihara, K., Kitagawa, T., Wada, S., & Yokogoshi, H. (2008). Effect of dietary docosahexaenoic acid connecting phospholipids on the lipid peroxidation of the brain in mice. Journal of Nutritional Science and Vitaminology, 54, 501–506.
Hiratsuka, S., Koizumi, K., Ooba, T., & Yokogoshi, H. (2009). Effects of dietary docosahexaenoic acid connecting phospholipids on the learning ability and fatty acid composition of the brain. Journal of Nutritional Science and Vitaminology, 55, 374–380.
Tang, X., Li, Z. J., Xu, J., Xue, Y., Li, J. Z., Wang, J. F., Yanagita, T., Xue, C. H., & Wang, Y. M. (2012). Short term effects of different omega-3 fatty acid formulation on lipid metabolism in mice fed high or low fat diet. Lipids in Health and Disease, 11, 70.
Rossmeisl, M., Jilkova, Z. M., Kuda, O., Jelenik, T., Medrikova, D., Stankova, B., Kristinsson, B., Haraldsson, G. G., Svensen, H., & Stoknes, I. (2012). Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: possible role of endocannabinoids. PloS One, 7, e38834.
Awada, M., Meynier, A., Soulage, C. O., Hadji, L., Géloën, A., Viau, M., Ribourg, L., Benoit, B., Debard, C., & Guichardant, M. (2013). N-3 PUFA added to high-fat diets affect differently adiposity and inflammation when carried by phospholipids or triacylglycerols in mice. Nutrition & Metabolism (London), 10, 23.
Vigerust, N. F., Bjørndal, B., Bohov, P., Brattelid, T., Svardal, A., & Berge, R. K. (2012). Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. European Journal of Nutrition, 52, 1315–1325.
Burri, L., Hoem, N., Banni, S., & Berge, K. (2012). Marine omega-3 phospholipids: metabolism and biological activities. International Journal of Molecular Sciences, 13, 15401–15419.
Vikbjerg, A. F., Mu, H., & Xu, X. (2005). Parameters affecting incorporation and by-product formation during the production of structured phospholipids by lipase-catalyzed acidolysis in solvent-free system. Journal of Molecular Catalysis B: Enzymatic, 36, 14–21.
Kim, I. H., Garcia, H. S., & Hill Jr., C. G. (2010). Synthesis of structured phosphatidylcholine containing n-3 PUFA residues via acidolysis mediated by immobilized phospholipase A1. Journal of the American Oil Chemists’ Society, 87, 1293–1299.
Lyberg, A. M., Adlercreutz, D., & Adlercreutz, P. (2005). Enzymatic and chemical synthesis of phosphatidylcholine regioisomers containing eicosapentaenoic acid or docosahexaenoic acid. European Journal of Lipid Science and Technology, 107, 279–290.
Schmid, A., Dordick, J., Hauer, B., Kiener, A., Wubbolts, M., & Witholt, B. (2001). Industrial biocatalysis today and tomorrow. Nature, 409, 258–268.
Sharma, S., Yadav, N., Chowdhury, P. K., & Ganguli, A. K. (2015). Controlling the microstructure of reverse micelles and their templating effect on shaping nanostructures. The Journal of Physical Chemistry B, 119, 11295–11306.
Hossen, M., & Hernandez, E. (2005). Enzyme-catalyzed synthesis of structured phospholipids with conjugated linoleic acid. European Journal of Lipid Science and Technology, 107, 730–736.
Yamamoto, Y., Mizuta, E., Ito, M., Harata, M., Hiramoto, S., & Hara, S. (2014). Lipase-catalyzed preparation of phospholipids containing n-3 polyunsaturated fatty acids from soy phospholipids. Journal of Oleo Science, 63, 1275–1281.
Hong, S. C., Park, K. M., Son, Y. H., Jung, H. S., Kim, K., Choi, S. J., & Chang, P. S. (2015). AOT/isooctane reverse micelles with a microaqueous core act as protective shells for enhancing the thermal stability of Chromobacterium viscosum lipase. Food Chemistry, 179, 263–269.
Chi, Z. Y., Hu, B., Liu, Y., Frear, C., Wen, Z. Y., & Chen, S. L. (2007). Production of ω-3 polyunsaturated fatty acids from cull potato using an algae culture process. Applied Biochemistry and Biotechnology, 137, 805–815.
Zhang, Y., Min, Q. S., Xu, J., Zhang, K., Chen, S. L., Wang, H. J., & Li, D. M. (2016). Effect of malate on docosahexaenoic acid production from Schizochytrium sp. B4D1. Electronic Journal of Biotechnology, 19, 56–60.
Chen, W. X, Wang, H. J, Zhang, K., Gao, F., Chen, S. L., & Li, D. M. (2016). Physicochemical properties and storage stability of microencapsulated DHA-rich oil with different wall materials. Applied Biochemistry and Biotechnology, 1–14.
Liu, B., Liu, J., Sun, P. P., Ma, X. N., Jiang, Y., & Chen, F. (2015). Sesamol enhances cell growth and the biosynthesis and accumulation of docosahexaenoic acid in the microalga Crypthecodinium cohnii. Journal of Agricultural and Food Chemistry, 63, 5640–5645.
Heck, J. X., Flôres, S. H., Hertz, P. F., & Ayub, M. A. Z. (2005). Optimization of cellulase-free xylanase activity produced by Bacillus coagulans BL69 in solid-state cultivation. Process Biochemistry, 40, 107–112.
Adlercreutz, D., Budde, H., & Wehtje, E. (2002). Synthesis of phosphatidylcholine with defined fatty acid in the sn-1 position by lipase-catalyzed esterification and transesterification reaction. Biotechnology and Bioengineering, 78, 403–411.
Egger, D., Wehtje, E., & Adlercreutz, P. (1997). Characterization and optimization of phospholipase A 2 catalyzed synthesis of phosphatidylcholine. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 1343, 76–84.
Hakoda, M., Shiragami, N., Enomoto, A., & Nakamura, K. (2003). Measurements of hydrodynamic diameter of AOT reverse micelles containing lipase in supercritical ethane and its enzymatic reaction. Bioprocess and Biosystems Engineering, 25, 243–247.
Haraldsson, G. G., & Thorarensen, A. (1999). Preparation of phospholipids highly enriched with n-3 polyunsaturated fatty acids by lipase. Journal of the American Oil Chemists’ Society, 76, 1143–1149.
Li, D. M., Qin, X. L., Wang, W. F., Li, Z. G., Yang, B., & Wang, Y. H. (2016). Synthesis of DHA/EPA-rich phosphatidylcholine by immobilized phospholipase A1: effect of water addition and vacuum condition. Bioprocess and Biosystems Engineering, 39, 1305–1314.
Kim, I. H., Garcia, H. S., & Hill, C. G. (2007). Phospholipase a 1-catalyzed synthesis of phospholipids enriched in n−3 polyunsaturated fatty acid residues. Enzyme and Microbial Technology, 40, 1130–1135.
Menger, F. M., & Yamada, K. (1979). Enzyme catalysis in water pools. Journal of the American Chemical Society, 101, 6731–6734.
Petersen, S. B., Jonson, P. H., Fojan, P., Petersen, E. I., Petersen, M. T. N., Hansen, S., Ishak, R. J., & Hough, E. (1998). Protein engineering the surface of enzymes. Journal of Biotechnology, 66, 11–26.
Kim, J., Lee, C. S., Oh, J., & Kim, B. G. (2001). Production of egg yolk lysolecithin with immobilized phospholipase A2. Enzyme and Microbial Technology, 29, 587–592.
Xu, X. (2000). Production of specific-structured triacylglycerols by lipase-catalyzed reactions: a review. European Journal of Lipid Science and Technology, 102, 287–303.
Rosu, R., Yasui, M., Iwasaki, Y., & Yamane, T. (1999). Enzymatic synthesis of symmetrical 1,3-diacylglycerols by direct esterification of glycerol in solvent-free system. Journal of the American Oil Chemists’ Society, 76, 839–843.
Virto, C., Svensson, I., & Adlercreutz, P. (1999). Enzymatic synthesis of lysophosphatidic acid and phosphatidic acid. Enzyme and Microbial Technology, 24, 651–658.
Kim, J., & Kim, B. G. (2000). Lipase-catalyzed synthesis of lysophosphatidylcholine using organic cosolvent for in situ water activity control. Journal of the American Oil Chemists’ Society, 77, 791–797.
Virto, C., & Adlercreutz, P. (2000). Lysophosphatidylcholine synthesis with Candida antarctica lipase B (Novozym 435). Enzyme and Microbial Technology, 26, 630–635.
Park, C. W., Kwon, S. J., Han, J. J., & Rhee, J. S. (2000). Transesterification of phosphatidylcholine with eicosapentaenoic acid ethyl ester using phospholipase A2 in organic solvent. Biotechnology Letters, 22, 147–150.
Peng, L., Xu, X., Mu, H., Høy, C. E., & Adler-Nissen, J. (2002). Production of structured phospholipids by lipase-catalyzed acidolysis: optimization using response surface methodology. Enzyme and Microbial Technology, 31, 523–532.
Xi, X., Feng, X. M., Shi, N. R., Ma, X. X., Lin, H., & Han, Y. Q. (2016). Immobilized phospholipase A1-catalyzed acidolysis of phosphatidylcholine from Antarctic krill (Euphausia superba) for docosahexaenoic acid enrichment under supercritical conditions. Journal of Molecular Catalysis B: Enzymatic, 126, 46–55.
Acknowledgements
This study was supported by the International Cooperation Project (grant no. 2014DFA61040), the Youth Innovation Promotion Association CAS, and the Hi-Tech Research and Development Program (863) of China (grant no. 2014ARA021701).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Guo, W., Gao, F. et al. Phospholipase A1-Catalysed Synthesis of Docosahexaenoic Acid-Enriched Phosphatidylcholine in Reverse Micelles System. Appl Biochem Biotechnol 182, 1037–1052 (2017). https://doi.org/10.1007/s12010-016-2379-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2379-y