Abstract
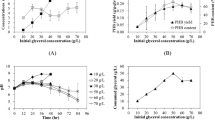
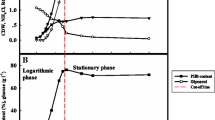
Recently, poly(3-hydroxybutyrate) (PHB) has been found in a few thermophilic strains where several advantages can be gained from running fermentation at high temperatures. Caldimonas manganoxidans, a thermophilic gram-negative bacterium, was investigated for the feasibility as a PHB-producing strain. It is suggested that the best fermentation strategy for achieving the highest PHB concentration of 5.4 ± 1.1 g/L (from 20 g/L glucose) in 24 h is to use the fermentation conditions that are favored for the bacterial growth, yet temperature and pH should be chosen at conditions that are favored for the PHB content. Besides, the above fermentation conditions produce PHB that has a high molecular weight of 1274 kDa with a low polydispersity index (PDI) of 1.45, where the highest Mw of PHB of 1399 kDa (PDI of 1.32) is obtained in this study. To the best knowledge of authors, C. manganoxidans has the best PHB productivity among the thermophiles and is comparable to those common PHB-producing mesophiles.









Similar content being viewed by others
References
Akar, A., Akkaya, E. U., Yesiladali, S. K., Çelikyilmaz, G., Çokgor, E. U., Tamerler, C., Orhon, D., & Çakar, Z. P. (2006). Accumulation of polyhydroxyalkanoates by Microlunatus phosphovorus under various growth conditions. Journal of Industrial Microbiology and Biotechnology, 33, 215–220.
Aoyagi, Y., Doi, Y., & Iwata, T. (2003). Mechanical properties and highly ordered structure of ultra-high-molecular-weight poly[(R)-3-hydroxybutyrate] films: effects of annealing and two-step drawing. Polymer Degradation and Stability, 79, 209–216.
Ashby, R. D., Solaiman, D. K., & Foglia, T. A. (2004). Bacterial poly (hydroxyalkanoate) polymer production from the biodiesel co-product stream. Journal of Polymer and the Environment, 12, 105–112.
Belal, E. B. (2013) Production of poly-βhydroxybutyric acid (PHB) by Rhizobium elti and Pseudomonas stutzeri. Current Research Journal of Biological Sciences, 5, 273–284.
Bertrand, J.-L., Ramsay, B., Ramsay, J., & Chavarie, C. (1990). Biosynthesis of poly-β-hydroxyalkanoates from pentoses by Pseudomonas pseudoflava. Applied and Environmental Microbiology, 56, 3133–3138.
Bonartsev, A., Myshkina, V., Nikolaeva, D., Furina, E., Makhina, T., Livshits, V., Boskhomdzhiev, A., Ivanov, E., Iordanskii, A., & Bonartseva, G. (2007). Biosynthesis, biodegradation, and application of poly (3-hydroxybutyrate) and its copolymers-natural polyesters produced by diazotrophic bacteria. Communicating Current Research and Educational Topics and Trends in Applied Microbiology, 1, 295–307.
Brigham, C. J., & Sinskey, A. J. (2012). Applications of polyhydroxyalkanoates in the medical industry. International Journal of Biotechnology for Wellness Industries, 1, 52–60.
Chen, S.-K., Chin, W.-C., Tsuge, K., Huang, C.-C., & Li, S.-Y. (2013). Fermentation approach for enhancing 1-butanol production using engineered butanologenic Escherichia coli. Bioresource Technology, 145, 204–209.
Chen, Y., Tsai, Y.-H., Chou, I., Tseng, S.-H. and Wu, H.-S. (2014) Application of biodegradable polyhydroxyalkanoates as surgical films for ventral hernia repair in mice. International Journal of Polymer Science, 11. doi:10.1155/2014/789681.
Chien, C.-C., Chen, C.-C., Choi, M.-H., Kung, S.-S., & Wei, Y.-H. (2007). Production of poly-β-hydroxybutyrate (PHB) by Vibrio spp. isolated from marine environment. Journal of Biotechnology, 132, 259–263.
Chien, C.-C., Hong, C.-C., Soo, P.-C., Wei, Y.-H., Chen, S.-Y., Cheng, M.-L., & Sun, Y.-M. (2010). Functional expression of phaCAB genes from Cupriavidus taiwanensis strain 184 in Escherichia coli for polyhydroxybutyrate production. Applied Biochemistry and Biotechnology, 162, 2355–2364.
Ciou, C.-Y., Li, S.-Y., & Wu, T.-M. (2014). Morphology and degradation behavior of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/layered double hydroxides composites. European Polymer Journal, 59, 136–143.
Grothe, E., Moo-Young, M., & Chisti, Y. (1999). Fermentation optimization for the production of poly (β-hydroxybutyric acid) microbial thermoplastic. Enzyme and Microbial Technology, 25, 132–141.
Hamieh, A., Olama, Z., & Holail, H. (2013). Microbial production of polyhydroxybutyrate, a biodegradable plastic using agro-industrial waste products. Global Advanced Research Journal of Microbiology, 2, 54–64.
Harding, K., Dennis, J., Von Blottnitz, H., & Harrison, S. (2007). Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. Journal of Biotechnology, 130, 57–66.
Ho, M.-H., Li, S.-Y., Ciou, C.-Y. and Wu, T.-M. (2014) The morphology and degradation behavior of electrospun poly(3-hydroxybutyrate)/Magnetite and poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Magnetite composites. Journal of Applied Polymer Science, 131, 41070.
Holst, O., Manelius, Å., Krahe, M., Märkl, H., Raven, N., & Sharp, R. (1997). Thermophiles and fermentation technology. Comparative Biochemistry and Physiology. Part A, Physiology, 118, 415–422.
Hoshino, A., & Isono, Y. (2002). Degradation of aliphatic polyester films by commercially available lipases with special reference to rapid and complete degradation of poly (L-lactide) film by lipase PL derived from Alcaligenes sp. Biodegradation, 13, 141–147.
Ibrahim, M., Willems, A., & Steinbüchel, A. (2010). Isolation and characterization of new poly (3HB)‐accumulating star‐shaped cell‐aggregates‐forming thermophilic bacteria. Journal of Applied Microbiology, 109, 1579–1590.
Jackson, F. A., & Dawes, E. A. (1976). Regulation of the tricarboxylic acid cycle and poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii grown under nitrogen or oxygen limitation. Journal of General Microbiology, 97, 303–312.
Kadouri, D., Jurkevitch, E., & Okon, Y. (2003). Involvement of the reserve material poly-β-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Applied and Environmental Microbiology, 69, 3244–3250.
Khanna, S., & Srivastava, A. K. (2005). Statistical media optimization studies for growth and PHB production by Ralstonia eutropha. Process Biochemistry, 40, 2173–2182.
Lawrence, A., Schoenheit, J., He, A., Tian, J., Liu, P., Stubbe, J., & Sinskey, A. (2005). Transcriptional analysis of Ralstonia eutropha genes related to poly-(R)-3-hydroxybutyrate homeostasis during batch fermentation. Applied Microbiology and Biotechnology, 68, 663–672.
Li, S.-Y., Srivastava, R., Suib, S. L., Li, Y., & Parnas, R. S. (2011). Performance of batch, fed-batch, and continuous A–B–E fermentation with pH-control. Bioresource Technology, 102, 4241–4250.
Liu, Y., Huang, S., Zhang, Y., & Xu, F. (2014). Isolation and characterization of a thermophilic Bacillus shackletonii K5 from a biotrickling filter for the production of polyhydroxybutyrate. Journal of Environmental Sciences, 26, 1453–1462.
Loh, X. J., Goh, S. H., & Li, J. (2007). Hydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly[(R)-3-hydroxybutyrate], poly(ethylene glycol), and poly(propylene glycol). Biomaterials, 28, 4113–4123.
Maehara, A., Ueda, S., Nakano, H., & Yamane, T. (1999). Analyses of a polyhydroxyalkanoic acid granule-associated 16-Kilodalton Protein and its putative regulator in the pha Locus of Paracoccus denitrificans. Journal of Bacteriology, 181, 2914–2921.
Miller, G. (1959). Use of DNS reagent for the measurement of reducing sugar. Analytical Chemistry, 31, 426–428.
Mona, K. G., Azza, E. S., & Sanaa, H. O. (2001). Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen source. Microbiological Research, 156, 201–207.
Nishioka, M., Nakai, K., Miyake, M., Asada, Y., & Taya, M. (2001). Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnology Letters, 23, 1095–1099.
Oehmen, A., Keller-Lehmann, B., Zeng, R. J., Yuan, Z., & Keller, J. (2005). Optimisation of poly-β-hydroxyalkanoate analysis using gas chromatography for enhanced biological phosphorus removal systems. Journal of Chromatography A, 1070, 131–136.
Philip, S., Sengupta, S., Keshavarz, T., & Roy, I. (2009). Effect of impeller speed and pH on the production of poly (3-hydroxybutyrate) using Bacillus cereus SPV. Biomacromolecules, 10, 691–699.
Quagliano, J., & Miyazaki, S. S. (1997). Effect of aeration and carbon/nitrogen ratio on the molecular mass of the biodegradable polymer poly-β-hydroxybutyrate obtained from Azotobacter chroococcum 6B. Applied Microbiology and Biotechnology, 48, 662–664.
Quillaguamán, J., Doan-Van, T., Guzmán, H., Guzmán, D., Martín, J., Everest, A., & Hatti-Kaul, R. (2008). Poly (3-hydroxybutyrate) production by Halomonas boliviensis in fed-batch culture. Applied Microbiology and Biotechnology, 78, 227–232.
Ramsay, B. A., Saracovan, I., Ramsay, J., & Marchessault, R. (1992). Effect of nitrogen limitation on long-side-chain poly-beta-hydroxyalkanoate synthesis by Pseudomonas resinovorans. Applied and Environmental Microbiology, 58, 744–746.
Santhanam, A., & Sasidharan, S. (2010). Microbial production of polyhydroxy alkanotes (PHA) from Alcaligens spp. and Pseudomonas oleovorans using different carbon sources. African Journal of Biotechnology, 9, 3144–3150.
Senior, P., Beech, G., Ritchie, G., & Dawes, E. (1972). The role of oxygen limitation in the formation of poly-beta-hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. The Biochemical Journal, 128, 1193–1201.
Shah, A. A., Hasan, F., Hameed, A., & Ahmed, S. (2008). Biological degradation of plastics: a comprehensive review. Biotechnology Advances, 26, 246–265.
Sheu, D.-S., Chen, W.-M., Yang, J.-Y., & Chang, R.-C. (2009). Thermophilic bacterium Caldimonas taiwanensis produces poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzyme and Microbial Technology, 44, 289–294.
Sim, S. J., Snell, K. D., Hogan, S. A., Stubbe, J., Rha, C., & Sinskey, A. J. (1997). PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nature Biotechnology, 15, 63–67.
Sorrentino, A., Gorrasi, G., & Vittoria, V. (2007). Potential perspectives of bio-nanocomposites for food packaging applications. Trends in Food Science and Technology, 18, 84–95.
Suzuki, T., Deguchi, H., Yamane, T., Shimizu, S., & Gekko, K. (1988). Control of molecular weight of poly-β-hydroxybutyric acid produced in fet-batch culture of Protomonas extorquens. Applied Microbiology and Biotechnology, 27, 487–491.
Taidi, B., Anderson, A. J., Dawes, E. A., & Byrom, D. (1994). Effect of carbon source and concentration on the molecular mass of poly (3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus. Applied Microbiology and Biotechnology, 40, 786–790.
Takeda, M., Kamagata, Y., Ghiorse, W. C., Hanada, S., & Koizumi, J.-i. (2002). Caldimonas manganoxidans gen. nov., sp. nov., a poly (3-hydroxybutyrate)-degrading, manganese-oxidizing thermophile. International Journal of Systematic and Evolutionary Microbiology, 52, 895–900.
Takeda, M., Kitashima, K., Adachi, K., Hanaoka, Y., Suzuki, I., & Koizumi, J.-i. (2000). Cloning and expression of the gene encoding thermostable poly (3-hydroxybutyrate) depolymerase. Journal of Bioscience and Bioengineering, 90, 416–421.
Takeda, M., Koizumi, J.-I., Yabe, K., & Adachi, K. (1998). Thermostable poly(3-hydroxybutyrate) depolymerase of a thermophilic strain of Leptothrix sp. isolated from a hot spring. Journal of Fermentation and Bioengineering, 85, 375–380.
Tanadchangsaeng, N., & Yu, J. (2012). Microbial synthesis of polyhydroxybutyrate from glycerol: gluconeogenesis, molecular weight and material properties of biopolyester. Biotechnology and Bioengineering, 109, 2808–2818.
Tokiwa, Y., & Calabia, B. (2006). Biodegradability and biodegradation of poly(lactide). Applied Microbiology and Biotechnology, 72, 244–251.
Trainer, M. A., & Charles, T. C. (2006). The role of PHB metabolism in the symbiosis of rhizobia with legumes. Applied Microbiology and Biotechnology, 71, 377–386.
Tseng, C.-L., Chen, H.-J., & Shaw, G.-C. (2006). Identification and characterization of the Bacillus thuringiensis phaZ gene, encoding new intracellular poly-3-hydroxybutyrate depolymerase. Journal of Bacteriology, 188, 7592–7599.
Valappil, S. P., Misra, S. K., Boccaccini, A. R., Keshavarz, T., Bucke, C., & Roy, I. (2007). Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. Journal of Biotechnology, 132, 251–258.
Vishnuvardhan Reddy, S., Thirumala, M., & Mahmood, S. K. (2009). Production of PHB and P (3HB-co-3HV) biopolymers by Bacillus megaterium strain OU303A isolated from municipal sewage sludge. World Journal of Microbiology and Biotechnology, 25, 391–397.
Wang, F., & Lee, S. Y. (1997). Poly (3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of alcaligenes latus under nitrogen limitation. Applied and Environmental Microbiology, 63, 3703–3706.
Wang, J., & Yu, H.-Q. (2007). Biosynthesis of polyhydroxybutyrate (PHB) and extracellular polymeric substances (EPS) by Ralstonia eutropha ATCC 17699 in batch cultures. Applied Microbiology and Biotechnology, 75, 871–878.
Xin, J., Zhang, Y., Dong, J., Song, H., & Xia, C. (2013). An experimental study on molecular weight of poly-3-hydroxybutyrate (PHB) accumulated in Methylosinus trichosporium IMV 3011. African Journal of Biotechnology, 10, 7078–7087.
Xu, F., Huang, S., Liu, Y., Zhang, Y., & Chen, S. (2014). Comparative study on the production of poly (3-hydroxybutyrate) by thermophilic Chelatococcus daeguensis TAD1: a good candidate for large-scale production. Applied Microbiology and Biotechnology, 98, 3965–3974.
Acknowledgments
This work was funded by the Ministry of Science and Technology Taiwan, MOST-102-2221-E-005-064 and MOST-103-2221-E-005-072-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsiao, LJ., Lin, JH., Sankatumvong, P. et al. The Feasibility of Thermophilic Caldimonas manganoxidans as a Platform for Efficient PHB Production. Appl Biochem Biotechnol 180, 852–871 (2016). https://doi.org/10.1007/s12010-016-2138-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2138-0