Abstract
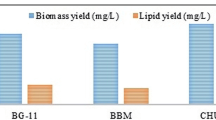
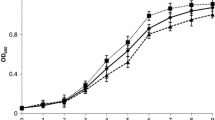
Algal biomass is gaining importance for biofuel production as it is rich in lipids. It becomes more significant when biomass is produced by capturing atmospheric greenhouse gas, CO2. In the present study, the effect of different physicochemical parameters were studied on the biomass and lipid productivity in Chlorella sp. MJ 11/11. The different parameters viz. initial pH, nitrate concentration, and phosphate concentration were optimized using single-parameter studies. The interactions between the parameters were determined statistically using the Box-Behnken design of optimization. The optimal values were decided by analyzing them with response surface methodology. The optimum levels of the parameters (pH 6.5, nitrate concentration 0.375 g L−1, and phosphate concentration 0.375 mL L−1) yielded a maximum biomass concentration of 1.26 g L−1 at a constant light intensity of 100 μmol m−2 s−1 and temperature of 30 °C. The effect of CO2 concentration on the biomass production was also investigated and was found to be a maximum of 4 g L−1 at 5 % air-CO2 mixture (v/v). Maximum lipid content of 24.6 % (w/w) was observed at 2 % air-CO2 mixture (v/v). Fatty acid analyses of the obtained algal biomass suggested that they could be a suitable feedstock for biodiesel production.




Similar content being viewed by others
References
Demirbas, A. (2007). Progress and recent trends in biofuels. Progress in Energy and Combustion Science, 33(1), 1–18.
Nayak, B. K., Pandit, S., Das. D., Biohydrogen. (2013). In: C., Kennes C., Veiga ría (Eds.), Air Pollut. Prev. Control (pp. 345–81). John Wiley & Sons, Ltd.
Ngangkham, M., Ratha, S. K., Prasanna, R., Saxena, A. K., Dhar, D. W., Sarika, C., et al. (2012). Biochemical modulation of growth, lipid quality and productivity in mixotrophic cultures of Chlorella sorokiniana. Springer Plus, 1, 33.
Becker, W. (2004). Microalgae in human and animal nutrition. In A. Richmond (Ed.), Handbook of microalgal culture (pp. 312–351). Oxford: Blackwell.
Chisti, Y. (2008). Biodiesel from microalgae beats bioethanol. Trends in Biotechnology, 26, 126–131.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Behrens, P. W., & Kyle, D. J. (1996). Microalgae as a source of fatty acids. Journal of Food Lipids, 3, 259–272.
Chaichalerm, S., Pokethitiyook, P., Yuan, W., Meetam, M., Sritong, K., Pugkaew, W., et al. (2012). Culture of microalgal strains isolated from natural habitats in Thailand in various enriched media. Applied Energy, 89, 296–302.
Barsanti, L., & Gualtieri, P. (2006). Algae: Anatomy, Biochemistry, and Biotechnology. Boca Raton: Taylor & Francis.
Karemore, A., Pal, R., & Sen, R. (2013). Strategic enhancement of algal biomass and lipid in Chlorococcum infusionum as bioenergy feedstock. Algal Research, 2(2), 113–121.
Roy, S., Ghosh, S., & Das, D. (2012). Improvement of hydrogen production with thermophilic mixed culture from rice spent wash of distillery industry. International Journal of Hydrogen Energy, 37, 15867–15874.
Roy, S., Vishnuvardhan, M., & Das, D. (2013). Improvement of hydrogen production by newly isolated Thermoanaerobacterium thermosaccharolyticum IIT BT-ST1. International Journal of Hydrogen Energy. doi:10.1016/j.ijhydene.2013.06.128.
Ferreira, S. L. C., Bruns, R. E., Ferreira, H. S., Matos, G. D., David, J. M., Brandão, G. C., et al. (2007). Box–Behnken design: an alternative for the optimization of analytical methods. Analytica Chimica Acta, 597, 179–186.
Azma, M., Mohamed, M. S., Mohamad, R., Rahim, R. A., & Ariff, A. B. (2011). Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochemical Engineering Journal, 53, 187–195.
Patil, P. D., Gude, V. G., Mannarswamy, A., Deng, S., Cooke, P., Munson-McGee, S., et al. (2011). Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresource Technology, 102, 118–122.
Li, Z. S., Yuan, H. L., Yang, J. S., & Li, B. Z. (2011). Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. Bioresource Technology, 102(19), 9128–9134.
Hu, Q. (2004). Environmental effects on cell composition. In A. Richmond (Ed.), Handbook of microalgal culture (pp. 312–351). Oxford: Blackwell.
Powell, N., Shilton, A., Pratt, S., & Chisti, Y. (2008). Factors Influencing Luxury Uptake of Phosphorus by Microalgae in Waste Stabilization Ponds. Environmental Science Technology, 42, 5958–5962.
Aizawa, K., & Miyachi, S. (1986). Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria. FEMS Microbiology Review, 39, 215–233.
Tsuzuki, M., & Miyachi, S. (1989). The function of carbonic anhydrase in aquatic photosynthesis. Aquatic Botany, 34, 85–104.
Aizawa, K., & Miyachi, S. (1984). Carbonic anhydrase located on cell surface increases the affinity for inorganic carbon in photosynthesis of Dunaliella tertiolecta. FEBS Letters, 173, 41–44.
Miyachi, S., Tsuzuki, M., Maruyama, I., Gantar, M., Miyachi, S., & Matsushima, H. (1986). Effects of CO2 concentration during growth on the intracellular structure of Chlorella and Scenedesmus. Journal of Phycology, 22, 313–319.
Tsuzuki, M., Gantar, M., Aizawa, K., & Miyachi, S. (1986). Ultrastructure of Dunaliella tertiolecta cells grown under low and high CO2 concentrations. Plant Cell Physiol, 27, 737–739.
Turpin, D. H., Miller, A. G., & Canvin, D. T. (1984). Carboxysome content of Synechococcus leopoliensis (Cyanophyta) in response to inorganic carbon. Journal of Phycology, 20, 249–253.
Box, G.E.P., Berkum, E.E.M.V. (1960). Some new three level designs for the study of quantitative variables.
Mopkar Anand, A. S. D., Sankar, V., & Daniel, D. K. (2013). Optimization of Light intensity, Nitrate concentration and Cultivation time for Biomass production by Chlorella minutissima using Response Surface Methodology. Journal of Applied Sciences Research, 9(1), 94–99.
Skjanes, K., Knutsen, G., Kallqvist, T., & Lindblad, P. (2008). H2 production from marine and freshwater species of green algae during sulfur deprivation and considerations for bioreactor design. International Journal of Hydrogen Energy, 33, 511–521.
Kumar, K., & Das, D. (2012). Growth characteristics of Chlorella sorokiniana in airlift and bubble column photobioreactors. Bioresource Technology, 116, 307–313.
Yadavalli, R., Rao, S. R., & Rao, C. S. (2013). Response surface methodological approach to optimize process parameters for the biomass production of Chlorella pyrenoidosa. International Journal of Biotechnology Research, 3(1), 37–48.
Zhang, H., Wang, W., Li, Y., Yang, W., & Shen, G. (2011). Mixotrophic cultivation of Botryococcus braunii. Biomass and Bioenergy, 35, 1710–1715.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Doan, T. T. Y., Sivaloganathan, B., & Obbard, J. P. (2011). Screening of marine microalgae for biodiesel feedstock. Biomass Bioenergy, 35, 2534–2544.
Dey, P., Banerjee, J., & Maiti, M. K. (2011). Comparative lipid profiling of two endophytic fungal isolates — Colletotrichum sp. and Alternaria sp. having potential utilities as biodiesel feedstock. Bioresource Technology, 102, 5815–5823.
Gimmler, H. (2001). Acidophilic and acidotolerant algae. In L. C. Rai & J. P. Gaur (Eds.), Algal Adaptation to Environmental Stresses Physiological, Biochemical and Molecular Mechanisms (pp. 259–290). Heidelberg: Springer Press.
Mandal, S., & Mallick, N. (2009). Microalga Scenedesmus obliquus as a potential source for biodiesel production. Applied Microbiology and Biotechnology, 84(2), 281–291.
Fried, S., Mackie, B., & Nothwehr, E. (2003). Nitrate and phosphate levels positively affect the growth of algae species found in Perry Pond. Tillers, 4, 21–24.
Fan, J., Cui, Y., Wan, M., Wang, W., & Li, Y. (2014). Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnology for Biofuels, 7, 17.
Fan, L. H., Zhang, Y. T., Zhang, L., & Chen, H. L. (2008). Evaluation of a membrane-sparged helical tubular photobioreactor for carbon dioxide biofixation by Chlorella vulgaris. Journal of Membrane Science, 325, 336–345.
Jacob-Lopes, E., Scoparo, C. H. G., & Franco, T. T., (2008). Rates of CO2 removal by Aphanothecemicroscopica Nageli in tubular photobioreactors. Chemical Engineering Progress, 47, 1365–1373.
Prathima Devi, M., & Venkata Mohan, S. (2012). CO2 supplementation to domestic wastewater enhances microalgae lipid accumulation under mixotrophic microenvironment: Effect of sparging period and interval. Bioresource Technology, 112, 116–123.
Zheng, H., Gao, Z., Yin, F., Ji, X., & Huang, H. (2012). Effect of CO2 supply conditions on lipid production of Chlorella vulgaris from enzymatic hydrolysates of lipid-extracted microalgal biomass residues. Bioresource Technology, 126, 24–30.
Demirbas, A. (2010). Biodiesel for future transportation energy needs. Energy Source Part A, 32(16), 1490–1508.
Yamaberi, K., Takagi, M., & Yoshida, T. (1998). Nitrogen depletion for intracellular triglyceride accumulation to enhance liquefaction yield of marine microalgal cells into a fuel oil. Journal of Marine Biotechnology, 6, 44–48.
Takagi, M., Karseno, S., & Yoshida, T. (2006). Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. Journal of Bioscience and Bioengineering, 101, 223–226.
Kong, W. B., Hua, S. F., Cao, H., Mu, Y. W., Yang, H., Song, H., et al. (2012). Optimization of mixotrophic medium components for biomass production and biochemical composition biosynthesis by Chlorella vulgaris using response surface methodology. Journal of the Taiwan Institute of Chemical Engineers, 43, 360–367.
Acknowledgments
The authors gratefully acknowledge University Grants Commission (UGC), and Council for Scientific and Industrial Research (CSIR) Govt. of India for fellowship; IIT Kharagpur and Department of Biotechnology (DBT), Govt. of India for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, S., Roy, S. & Das, D. Improvement of Biomass Production by Chlorella sp. MJ 11/11 for Use as a Feedstock for Biodiesel. Appl Biochem Biotechnol 175, 3322–3335 (2015). https://doi.org/10.1007/s12010-015-1503-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1503-8