Abstract
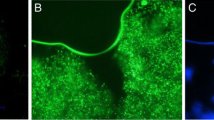
The main objective of this work was to obtain microencapsulated probiotic cells in order to improve their resistance to heat stress and gastrointestinal conditions. A further aim was to obtain a potentially probiotic chocolate soufflé. Lactobacillus reuteri DSM 17938 cells were microencapsulated by spray drying in alginate matrix and further coated with chitosan. Bacterial survival after exposure to different heat treatments and simulated gastrointestinal conditions were measured to test the microcapsules. They were also dyed by using a LIVE/DEAD® BacLight™ Bacterial Viability Kit and characterized by epifluorescence microscope observation. Furthermore, a potentially chocolate soufflé was prepared using microencapsulated cells. The results indicated that alginate microcapsules did not improve acid tolerance or heat resistance in “in vitro” experiments, while they were able to protect 7% of the Lactobacillus reuteri population during the baking of a chocolate soufflé, compared to a survival rate of 1% of free cells. By contrast, the cells microencapsulated with alginate coated with chitosan showed, compared to free cells, improved acid tolerance, allowing the cell population to remain constant after 3 h in simulated gastric conditions. Moreover, the heat resistance of cells in co-cross-linked microcapsules significantly improved compared to free cells, both in “in vitro” and “in food” experiments. Microencapsulation led to a survival rate of 10% after baking a chocolate soufflé. However, the final level of bacterial cells in the product was too low to consider the chocolate soufflé as a probiotic product.






Similar content being viewed by others
References
Anal, A. K., & Singh, H. (2007). Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends in Food Science and Technology, 18(5), 240–251.
Anal, A. K., & Stevens, W. F. (2005). Chitosan–alginate multilayer beads for controlled release of ampicillin. International Journal of Pharmaceutics, 290(1–2), 45–54.
Casas, I. A., & Dobrogosz, W. J. (2000). Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microbial Ecology in Health and Disease, 12(39), 247–285.
Cleusix, V., Lacroix, C., Vollenweider, S., & Le Blay, G. (2008). Glycerol induces reuterin production and decreases Escherichia coli population in an in vitro model of colonic fermentation with immobilized human feces. FEMS Microbiology Ecology, 63(1), 56–64.
Dommels, Y. E. M., Kemperman, R. A., Zebregas, Y. E. M. P., Draaisma, R. B., Jol, A., Wolvers, D. A. W., Vaughan, E. E., & Albers, R. (2009). Survival of Lactobacillus reuteri DSM 17938 and Lactobacillus rhamnosus GG in the human gastrointestinal tract with daily consumption of a low-fat probiotic spread. Applied and Environmental Microbiology, 75(19), 6198–6204.
Ercolini, D., La Storia, A., Villani, F., & Mauriello, G. (2006). Effect of a bacteriocin-activated polythene film on Listeria monocytogenes as evaluated by a viable staining and epifluorescence microscopy. Journal of Applied Microbiology, 100(4), 765–772.
Ercolini, D., Russo, F., Nasi, A., Ferranti, P., & Villani, F. (2009). Mesophilic and psychotropic bacteria from meat and their spoilage potential in vitro and in beef. Applied and Environmental Microbiology, 75(7), 1990–2001.
Gbassi, K. G., Vandamme, T., Ennahar, S., & Marchioni, E. (2009). Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey protein. International Journal of Food Microbiology, 129(1), 103–105.
Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Application of spray drying in microencapsulation of food ingredients: an overview. Food Research International, 40(9), 1107–1121.
Gibbs, F. B., Kermasha, S., Alli, I., & Mulligan, C. N. (1999). Encapsulation in the food industry. International Journal of Food Science and Nutrition, 50(3), 213–224.
Gombotz, W. R., & Wee, S. F. (1998). Protein release from alginate matrices. Advanced Drug Delivery Reviews, 31(3), 267–285.
Gouin, S. (2004). Microencapsulation: industrial appraisal of existing technologies and trends. Trends in Food Science and Technology, 15(7–8), 330–347.
Iannuccelli, V., Montanari, M., Bertelli, D., Pellati, F., & Coppi, G. (2011). Microparticulate polyelectolyte complexes for gentamicin transport across intestinal epithelia. Drug Delivery, 18(1), 26–37.
Kandler, O., Stetter, K. O., & Kohl, R. (1980). Lactobacillus reuteri sp. nov., a new species of heterofermentative lactobacilli. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene, 1(3), 264–269.
Koo, S. M., Cho, Y. H., Huh, C. S., Baek, Y. J., & Park, J. (2001). Improvement of the stability of Lactobacillus casei YIT 9018 by microencapsulation using alginate and chitosan. Journal of Microbiology and Biotechnology, 11(3), 376–383.
Krasaekoopt, W., Bhandari, B., & Deeth, H. (2004). The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. International Dairy Journal, 14(8), 737–743.
Lee, D. W., Hwang, S. J., Park, J. B., & Park, H. J. (2003). Preparation and release characteristics of polymer-coated and blended alginate microspheres. Journal of Microencapsulation, 20(2), 179–192.
Lee, J. S., Cha, D. S., & Park, H. J. (2004). Survival of freeze-dried Lactobacillus bulgaricus KFRI 673 in chitosan-coated calcium alginate microparticles. Journal of Agricultural and Food Chemistry, 52(24), 7300–7305.
Lertsutthiwong, P., Rojsitthisak, P., & Nimmannit, U. (2009). Preparation of turmeric oil-loaded chitosano–alginate biopolymeric nanocapsules. Materials Science and Engineering, 29(3), 856–860.
Lian, W. C., Hsiao, H. C., & Chou, C. C. (2002). Survival of bifidobacteria after spray drying. International Journal of Food Microbiology, 74(1–2), 79–86.
Mattila-Sandholm, T., Myllarinen, P., Crittenden, R., Mogensen, G., Fonden, R., & Saarela, M. (2002). Technological challenges for future probiotic food. International Dairy Journal, 12(2), 173–182.
Murugesan, R., & Orsat, V. (2011). Spray drying for the production of nutraceutical ingredients—a review. Food and Bioprocess Technology. doi:10.1007/s11947-011-0638-z.
Muthukumarasamy, P., Allan-Wojtas, P., & Holley, R. A. (2006). Stability of Lactobacillus reuteri in different type of microcapsules. Journal of Food Microbiology and Safety, 71(1), 20–24.
Reid, A. A., Champagne, C. P., Gardner, R., Fustier, P., & Vuillemard, J. C. (2007). Survival in food systems of Lactobacillus rhamnosus R011 microentrapped in whey protein gel particles. Journal of Food Microbiology and Safety, 72(1), 31–37.
Ribeiro, A. J., Neufeld, R. J., Arnaud, P., & Chaumeil, J. C. (1999). Microencapsulation of lipophilic drugs in chitosan-coated alginate microspheres. International Journal of Pharmaceutics, 187(1), 115–123.
Rokka, S., & Rantamaki, P. (2010). Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. European Food Research and Technology, 231(1), 1–12.
Rosander, A., Connolly, E., & Roos, S. (2008). Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17398. Applied and Environmental Microbiology, 74(19), 6032–6040.
Sabikhi, L., Babu, R., Thompkinson, D. K., & Kapila, S. (2010). Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food and Bioprocess Technology, 3, 586–593.
Serp, D., Mueller, M., von Stockar, U., & Marison, W. (2002). Low-temperature electron microscopy for the study of polysaccharide ultrastructure in hydrogels. II. Effect of temperature on the structure of Ca2+-alginate beads. Biotechnology and Bioengineering, 79(3), 253–259.
Smidsrod, O., & Skijak-Brack, G. (1990). Alginate as immobilization matrix for cells. Trends in Biotechnology, 8(3), 71–78.
Talarico, T. L., Casas, I. A., Chung, T. C., & Dobrogosz, W. J. (1988). Production and isolation of reuterin: a growth inhibitor produced by Lactobacillus reuteri. Antimicrobial Agents and Chemotherapy, 32(12), 1854–1858.
Tannock, G. W., & Archibald, R. D. (1984). The derivation and use of mice which do not harbor lactobacilli in the gastrointestinal tract. Canadian Journal of Microbiology, 30(6), 843–853.
Thu, B., Bruheim, P., Espevik, T., Smidsrod, O., Soon-Shiong, P., & Skijak-Brak, G. (1996). Alginate polycation microcapsules. I. Interaction between alginate and polycation. Biomaterials, 17(10), 1031–1040.
Zhao, R., Sun, J., Torley, P., Wang, D., & Niu, S. (2008). Measurement of particle diameter of Lactobacillus acidophilus microcapsule by spray drying and analysis on its microstructure. World Journal of Microbiology and Biotechnology, 24(8), 1349–1354.
Acknowledgments
This work was supported by a grant from the “Ministero dell'Istruzione, dell'Università e della Ricerca” within the program PON Research and Competitiveness 2007–2013. Project title: “Encapsulation of active ingredients for improving the quality and food safety”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malmo, C., La Storia, A. & Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Soufflé. Food Bioprocess Technol 6, 795–805 (2013). https://doi.org/10.1007/s11947-011-0755-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0755-8